Deep inside the cells of the brain, a small and often overlooked enzyme has been quietly playing a powerful role in keeping neurons alive. For years, scientists believed that the enzyme biliverdin reductase A—known as BVRA—had one main job: converting biliverdin into bilirubin, the yellow pigment responsible for the color of bruises and bile. But new research from Johns Hopkins Medicine reveals a far more extraordinary truth.
BVRA, it turns out, is much more than a pigment-producing enzyme. It is a guardian—one that helps protect brain cells from oxidative stress, one of the most damaging biological processes linked to aging and neurodegenerative diseases like Alzheimer’s.
Published in the Proceedings of the National Academy of Sciences (PNAS), this discovery offers an exciting new perspective on how the brain defends itself against decline and disease.
Unmasking BVRA’s Hidden Superpower
Oxidative stress is a silent enemy within our bodies. It occurs when harmful molecules called oxidants overwhelm the natural antioxidants that protect our cells. In the brain, where energy demand is high and oxygen flow constant, this imbalance can wreak havoc—damaging neurons, disrupting communication, and contributing to the slow unraveling seen in conditions like Alzheimer’s and Parkinson’s disease.
Bindu Paul, M.S., Ph.D., associate professor of pharmacology, psychiatry, and neuroscience at the Johns Hopkins University School of Medicine, has spent years investigating the molecular defenses that help neurons survive such stress. Her latest work uncovers that BVRA isn’t just a supporting player—it’s a key protector.
“Our research identifies BVRA as a key player in cellular defense with profound implications for aging, cognition and neurodegeneration,” Paul explains.
Her team discovered that BVRA’s protective power doesn’t depend on its well-known ability to produce bilirubin. Instead, BVRA works directly with another critical protein called NRF2, a master regulator of the cell’s antioxidant systems. Together, BVRA and NRF2 form a kind of biochemical partnership—one that strengthens the cell’s defenses and keeps oxidative damage at bay.
A Crucial Partnership Inside the Cell
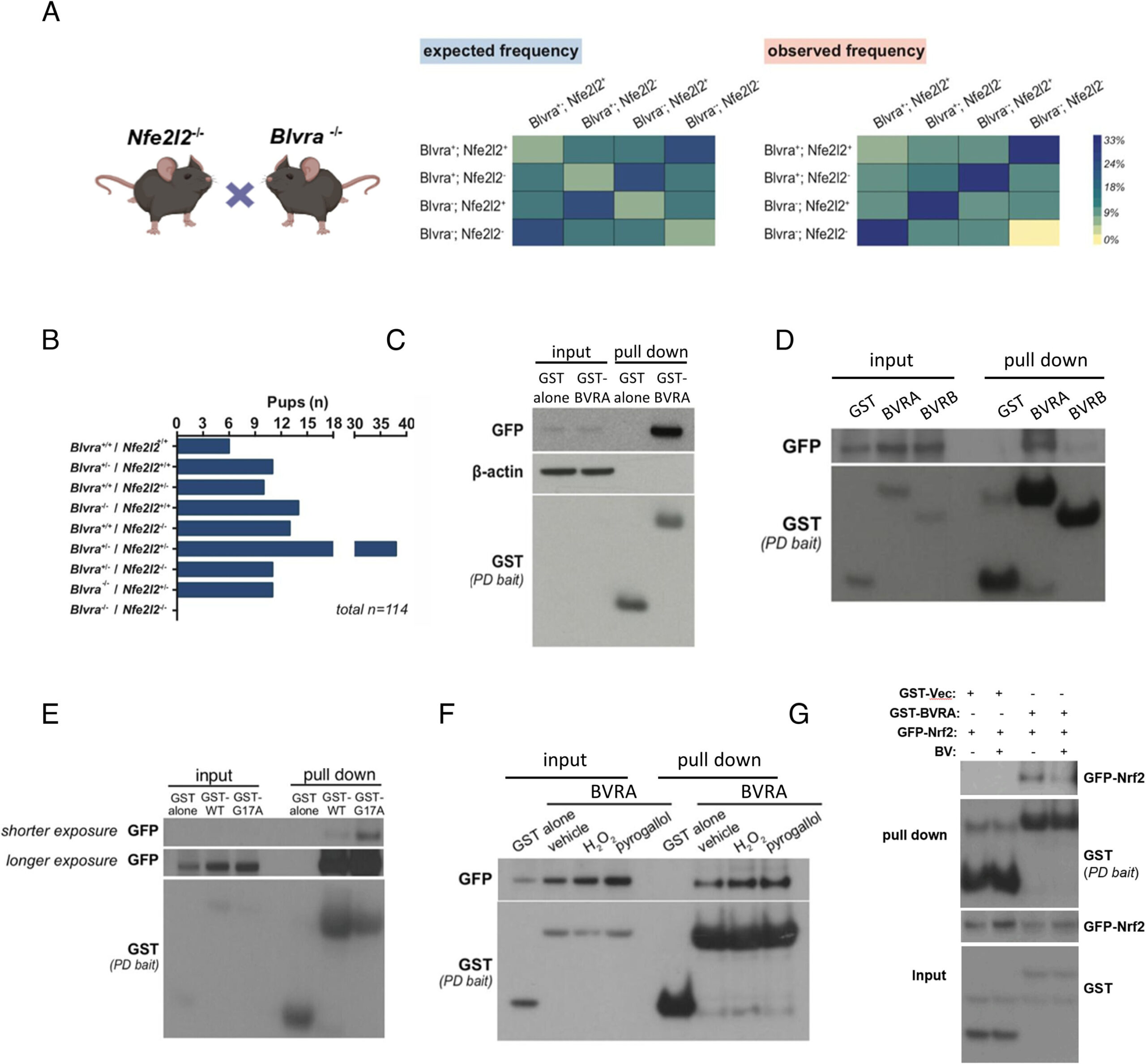
To explore BVRA’s connection to NRF2, the Johns Hopkins team conducted a series of sophisticated experiments using genetically engineered mice. These weren’t ordinary mice—they had been specifically designed to lack the genes that make either BVRA, NRF2, or both.
The results were striking. When the mice lacked both BVRA and NRF2, none of them survived. This revealed that the two proteins have a vital interaction—an interdependence essential for life itself.
Next, the researchers focused on mice missing only BVRA. Without it, NRF2 began to malfunction. The genes that NRF2 normally activates—those responsible for producing antioxidants and protective molecules—became sluggish, leaving the brain more vulnerable to oxidative stress.
The team went further, examining cultured brain cells to observe how BVRA and NRF2 interact. They found that the two physically bind to each other, working as partners to regulate genes involved in oxygen transport, immune signaling, and mitochondrial function.
“This work shows that BVRA does more than produce bilirubin, and is actually a molecular integrator of key cellular processes that help protect neurons from damage,” says first author Chirag Vasavda, M.D., Ph.D., a physician at Harvard Medical School and Massachusetts General Hospital who began the research as a Johns Hopkins M.D./Ph.D. student.
Beyond Pigments: A Molecular Guardian
Perhaps the most astonishing finding came when scientists tested whether BVRA could still protect neurons without making bilirubin. They created a mutated version of BVRA that lacked the ability to produce the pigment. Even without this function, the enzyme retained its ability to bind to NRF2 and activate the genes that shield cells from oxidative damage.
In other words, BVRA’s most critical role in brain health has nothing to do with color—it’s about control. It acts as a molecular switch, fine-tuning the cell’s antioxidant response system to maintain balance and survival under stress.
“This work highlights the long-term value of mechanistic discovery,” says co-first author Ruchita Kothari, a graduate student involved in the study. “It reminds us that even well-known enzymes can hold unexpected secrets when we look closer.”
Why This Matters for Alzheimer’s and Neurodegeneration
The implications of this discovery are profound. Oxidative stress is one of the earliest and most consistent hallmarks of neurodegenerative diseases. In Alzheimer’s, for instance, neurons are chronically exposed to oxidative damage caused by abnormal protein buildup and inflammation.
If BVRA helps to control oxidative stress by regulating NRF2 and antioxidant gene expression, then targeting BVRA’s pathway could open new therapeutic possibilities. Enhancing or mimicking BVRA’s function might strengthen neurons’ resilience against degeneration, slowing the progression of diseases that currently have no cure.
“This role of BVRA could potentially be targeted by drugs to slow the development of neurodegenerative disorders such as Alzheimer’s disease,” says Solomon H. Snyder, M.D., a co-corresponding author and distinguished professor of neuroscience, pharmacology, and psychiatry at Johns Hopkins.
Future studies, Paul adds, will examine how this BVRA–NRF2 partnership behaves in mouse models of Alzheimer’s. Understanding where and how it fails could point to entirely new strategies for preserving brain function as we age.
The Power of Collaboration and Discovery
Behind this breakthrough lies a story of persistence and teamwork. The study represents years of effort by scientists from multiple institutions, combining expertise in neuroscience, biochemistry, genomics, and clinical medicine.
“Our efforts underscore the power of multidisciplinary collaboration fueled by long-term investment in scientific research to address complex biological challenges,” Paul notes.
This collaboration allowed the team to move beyond isolated molecular questions and see the bigger picture—how one enzyme could influence the brain’s broader defense network. It’s a reminder that science often advances not through sudden eureka moments, but through patient, interconnected exploration.
Rewriting the Biology of the Brain
For decades, BVRA was seen as a minor character in the body’s biochemistry—a quiet enzyme performing a narrow function. This new research flips that assumption. Instead of being a simple pigment producer, BVRA emerges as a conductor in the orchestra of cellular protection, coordinating responses that help neurons survive the relentless stress of aging and disease.
“Our research identifies a vital non-canonical role of BVRA that plays key roles in neuronal signaling, which may be harnessed for therapeutic benefits,” Paul says.
That phrase—“non-canonical role”—captures the wonder of discovery. In science, it refers to something unexpected, a function outside the known rules. And in the case of BVRA, it reveals how much more there is to learn about even the most familiar molecules.
A New Hope for Protecting the Aging Brain
The study from Johns Hopkins is not just a technical advance; it’s a story of hope. It suggests that within the intricate machinery of the brain lies a built-in resilience, a molecular defense system that can be strengthened and supported.
In the future, drugs inspired by BVRA’s function might help maintain this resilience, slowing the onset of cognitive decline and neurodegeneration. The path to such treatments will take time, but discoveries like this one bring us closer to understanding how the brain protects itself—and how we might help it do so more effectively.
As researchers continue to map the dance between BVRA and NRF2, they are not only rewriting the biology of brain defense but also reaffirming a timeless truth: that even the smallest molecules can hold the biggest keys to life, memory, and the enduring mystery of the human mind.
More information: Chirag Vasavda et al, Biliverdin reductase A is a major determinant of protective NRF2 signaling, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2513120122