For decades, scientists believed that once the human heart was damaged, it could never truly heal. A heart attack meant permanent loss—cells that died could not be replaced, leaving scars that weakened the organ and shortened lives. But a groundbreaking discovery from Mount Sinai may be about to rewrite that story, bringing new hope to millions who suffer from heart disease.
A naturally occurring gene called Cyclin A2 (CCNA2), which lies dormant after birth, has shown the remarkable ability to regenerate heart cells when reactivated. This discovery, led by Dr. Hina Chaudhry, Director of Cardiovascular Regenerative Medicine at the Icahn School of Medicine at Mount Sinai, represents one of the most significant advances in cardiac science in recent memory. Published in npj Regenerative Medicine, the study suggests that human hearts—long thought incapable of repair—might one day heal themselves.
Awakening a Sleeping Power Within the Heart
From the moment we are born, our heart cells stop dividing. The organ that beats more than 100,000 times a day relies on the same finite number of muscle cells for an entire lifetime. When a heart attack strikes, millions of these cells die within minutes, and unlike skin or liver tissue, the heart cannot grow new ones. Instead, it forms scar tissue, which lacks the strength and flexibility of true muscle. This process is what leads to chronic heart failure, one of the leading causes of death worldwide.
Dr. Chaudhry’s team wanted to challenge that biological limit. They turned their attention to Cyclin A2, a gene that is active during fetal development when the heart is still growing. After birth, the gene switches off permanently, locking away the body’s natural ability to regenerate the heart. What would happen, the researchers wondered, if they could turn this gene back on in adults? Could they persuade the heart to repair itself?
Building on Two Decades of Research
This breakthrough didn’t happen overnight. It’s the culmination of nearly twenty years of work, beginning with a landmark 2014 study published in Science Translational Medicine. In that earlier research, Dr. Chaudhry’s team succeeded in regenerating the heart of a large mammal—a pig—after a heart attack by reactivating the CCNA2 gene. Because pig hearts are structurally and functionally similar to human hearts, this was a major step forward in regenerative medicine.
That earlier success demonstrated that it was possible, at least in theory, to restore a damaged heart using a naturally occurring gene. But there was still a long way to go. Scientists needed to show that the same approach could work in human heart cells—safely, effectively, and without causing harm.
Now, the new study bridges that critical gap, showing for the first time that adult human heart cells—long thought to be permanently incapable of dividing—can be coaxed into regeneration.
Turning Back the Clock on Human Heart Cells
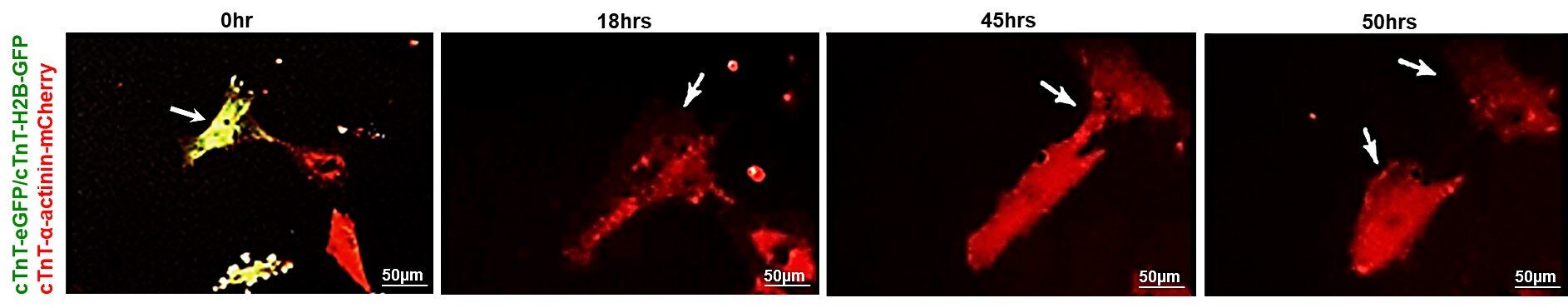
To test their theory, Dr. Chaudhry’s team created a replication-deficient viral vector, a specially designed delivery system that carries the CCNA2 gene into heart cells. Importantly, this viral vector was human-compatible, ensuring that it could be used safely in future clinical treatments.
They introduced this vector into living heart muscle cells taken from healthy donor hearts of adults aged 21, 41, and 55. Using advanced time-lapse imaging, they observed what happened when the gene was switched back on.
The results were astonishing. In the older donor hearts—those from the 41-year-old and the 55-year-old—the cells began to divide again, forming new, functioning heart muscle cells. These new “daughter” cells retained all the proper structures and calcium activity of normal cardiomyocytes, meaning they worked just like regular heart cells.
In contrast, cells from the 21-year-old heart showed little to no change, confirming what earlier research suggested: that younger hearts already possess some natural regenerative capacity and may not need CCNA2’s intervention.
What this means is that even middle-aged adult hearts, long past the age of growth or self-repair, can be reawakened to heal themselves with the help of this single gene.
A Gentle Reawakening, Not a Genetic Overhaul
One of the most remarkable aspects of this discovery is how precise and natural the process appears to be. Turning CCNA2 back on doesn’t transform heart cells into something unnatural or risky—it simply reactivates a dormant biological pathway that once allowed them to divide during early life.
Dr. Chaudhry’s team found that CCNA2 reawakens a series of growth genes temporarily, allowing cells to re-enter the cell cycle and divide, then return to their stable state. This is critical because uncontrolled cell growth, as seen in cancers or certain heart diseases, could be dangerous. But CCNA2’s effects are controlled and targeted—it encourages repair without making the heart tissue abnormally thick or dysfunctional.
In essence, this therapy doesn’t rebuild the heart by adding something foreign—it reminds the heart how to heal itself.
From Managing Disease to Curing It
For decades, medicine has focused on managing the symptoms of heart disease rather than reversing the damage it causes. Patients who suffer from heart attacks are given drugs to improve circulation, reduce strain, and prevent further injury. Those with severe heart failure may need implanted devices like pacemakers or even heart transplants.
But all these interventions treat the consequences, not the cause. None can replace the lost heart muscle that powers life itself.
“This shifts the paradigm from managing symptoms to actually repairing the human heart,” said Dr. Chaudhry. “Our goal is to deliver a therapy that allows the heart to heal itself after a heart attack or in heart failure—reducing the need for transplants or mechanical devices.”
If this therapy proves effective in human trials, it could change the entire field of cardiology. Rather than lifelong medications or invasive surgeries, patients might one day receive a one-time gene therapy that triggers their own hearts to regrow lost muscle—restoring strength, rhythm, and life.
Hope for the Future of Heart Regeneration
The potential impact of this discovery cannot be overstated. Heart disease is the leading cause of death worldwide, responsible for nearly 18 million deaths each year. Millions more live with heart failure, a chronic condition that robs them of energy, mobility, and independence.
For these individuals, CCNA2 therapy represents a new kind of hope—a future where heart damage might not be permanent, where recovery means full restoration rather than slow decline.
Dr. Chaudhry and her team are now preparing to take the next step: seeking FDA approval to begin clinical trials in patients. If successful, these trials could pave the way for the world’s first regenerative heart treatment based on reactivating a natural human gene.
Such trials will need to carefully test the safety, dosage, and long-term effects of the therapy, ensuring that it works as intended without unintended side effects. But the groundwork has been laid, and the early results are extraordinarily promising.
The Human Story Behind the Science
Beyond the data and genetic codes, there’s a deeply human story behind this discovery. For nearly twenty years, Dr. Hina Chaudhry has dedicated her career to one powerful idea: that the heart is not beyond repair. Her research, driven by both scientific vision and compassion, has pushed against decades of skepticism in the field.
In her own words, “This is the culmination of nearly two decades of work. We pioneered the concept that the heart could be regenerated by reawakening dormant cell division genes, and now we’ve brought that vision one step closer to patients.”
Her persistence, and the breakthroughs of her team, remind us that the boundaries of medicine are constantly being redrawn—not just by technology, but by human determination and imagination.