For decades, vaccines have been one of medicine’s most powerful defenses, training the immune system to recognize and defeat specific pathogens. The chickenpox vaccine, for instance, was designed solely to fight the varicella-zoster virus, and nothing else. This “one-pathogen, one-vaccine” model has been the standard approach for more than a century.
But the COVID-19 pandemic changed everything. The speed at which new variants emerged — some capable of slipping past vaccine protection — has sparked an urgent search for a new strategy: vaccines that don’t just protect against a single virus, but entire families of viruses, including dangerous strains we haven’t even seen yet.
“Our pipeline is challenging that approach,” says Dr. Alba Grifoni, Research Assistant Professor at the La Jolla Institute for Immunology (LJI). Her team’s latest work, published in Cell, lays the foundation for so-called universal vaccines — shots that could one day shield us against multiple viruses with pandemic potential.
The Science Behind a Universal Shield
To understand how a universal vaccine might work, you have to start with the fact that many viruses are related. COVID-19 is caused by SARS-CoV-2, which belongs to the coronavirus family. That family also includes viruses that cause common colds, as well as far deadlier members like MERS-CoV and the original SARS-CoV.
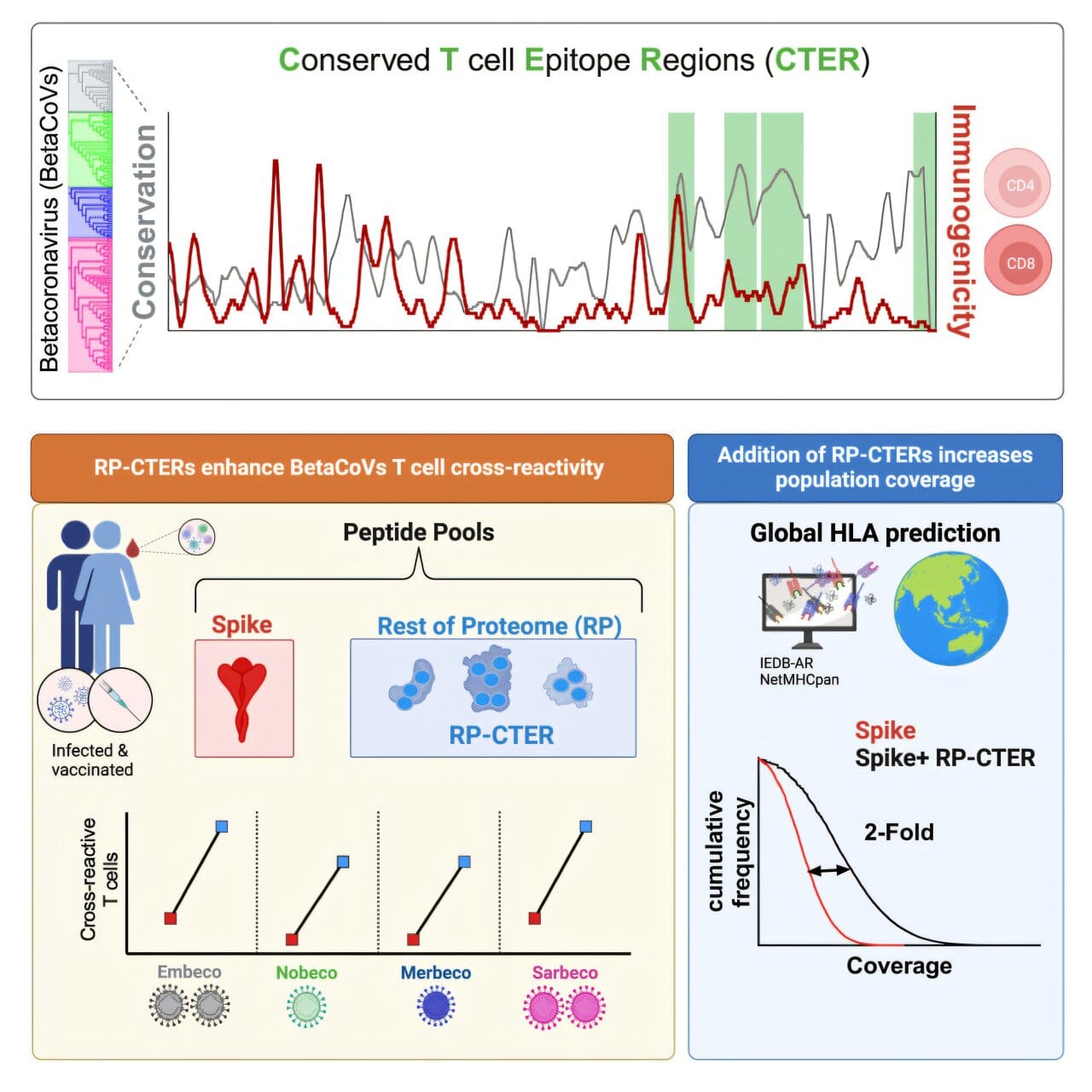
Despite their differences, these viruses share “conserved” protein sequences — molecular features that have remained unchanged over time, even as the viruses mutate. This is important because the immune system, particularly a type of white blood cell called the T cell, can recognize these conserved features. T cells don’t just attack one specific virus; they can identify and destroy infected cells from related viruses if those cells display the same conserved protein fragments, called epitopes.
Grifoni’s earlier research revealed that some T cells could detect these shared epitopes across both SARS-CoV-2 and common cold coronaviruses. That finding hinted at a tantalizing possibility: if scientists could map all the most important conserved epitopes, they could design vaccines that prepare T cells to respond broadly — not just to one virus, but to many.
“It is important to induce a neutralizing antibody response,” explains Grifoni, “but we’ve shown that T cells are much more stable in the context of viral variants. That’s because T cells look at all the proteins of the virus.”
Mining Data for Hidden Clues
Finding those key epitopes is no small task. While scientists already know some exist — including a few on the coronavirus spike protein — many more remain undiscovered, buried in complex immunology data.
To unearth them, Grifoni’s team turned to the Immune Epitope Database (IEDB), an open resource managed by LJI scientists. The database contains information on more than 200 coronavirus epitopes identified by researchers worldwide.
Working with virologists at the J. Craig Venter Institute, Grifoni’s group used bioinformatics and artificial intelligence to compare epitopes from a range of coronaviruses. They searched for hidden similarities, especially those found outside the spike protein — a critical step because spike proteins tend to mutate quickly. By focusing on more stable viral structures, they could identify targets less likely to change over time.
Their analysis revealed which epitopes sparked the strongest T cell responses, offering a roadmap for designing vaccines that could trigger broad and durable immunity.
Preparing for the Viruses We Haven’t Met Yet
The implications of this research go far beyond COVID-19. Grifoni envisions a future where this pipeline can be applied to multiple viral families — not just coronaviruses, but also respiratory pathogens like measles, Nipah virus, and enteroviruses (such as A71 and D68), as well as viruses that cause deadly hemorrhagic fevers like Lassa and Junin.
“The idea is that if a new coronavirus emerges, we might not be able to protect from the infection, but we might be able to protect from hospitalization,” says Grifoni. In other words, the first universal vaccines might not completely prevent illness — but they could stop the most severe outcomes, buying time for more targeted vaccines to be developed.
Bridging Knowledge Gaps
Right now, the path to universal vaccines is still in the research stage, but Grifoni believes the momentum is building. Her lab is already collaborating with teams studying multiple viral families, each with its own unique biology and pandemic risks.
“This coronavirus study shows the accuracy and usefulness of a new research pipeline,” she says. “We need to fill the knowledge gaps.”
In an age where the next pandemic could emerge at any time, that knowledge could mean the difference between a global crisis and a swiftly contained outbreak. By harnessing the power of T cells and the genetic commonalities among viruses, researchers like Grifoni are working to transform vaccine development — from fighting one enemy at a time to building an immune defense that can stand against many.
The science is still young, but its promise is vast. For the first time, we may be learning how to train our immune systems to fight not just the viruses we know, but the ones we’ve yet to meet.
More information: Tertuliano Alves Pereira Neto et al, Highly conserved Betacoronavirus sequences are broadly recognized by human T cells, Cell (2025). DOI: 10.1016/j.cell.2025.07.015