In a groundbreaking new study that could reshape how we understand and detect Alzheimer’s disease, scientists at Brown University have discovered a brain signal that may predict—years in advance—whether someone with mild cognitive impairment will go on to develop Alzheimer’s.
The study, published in Imaging Neuroscience, introduces a powerful new biomarker that emerges from electrical brain activity, offering clinicians a potential early-warning system for one of the world’s most devastating neurological conditions.
“We’ve detected a pattern in electrical signals of brain activity that predicts which patients are most likely to develop the disease within two and a half years,” said Dr. Stephanie Jones, professor of neuroscience at Brown University and co-leader of the research. “Being able to noninvasively observe a new early marker of Alzheimer’s disease progression in the brain for the first time is a very exciting step.”
Listening to the Brain’s Quiet Warnings
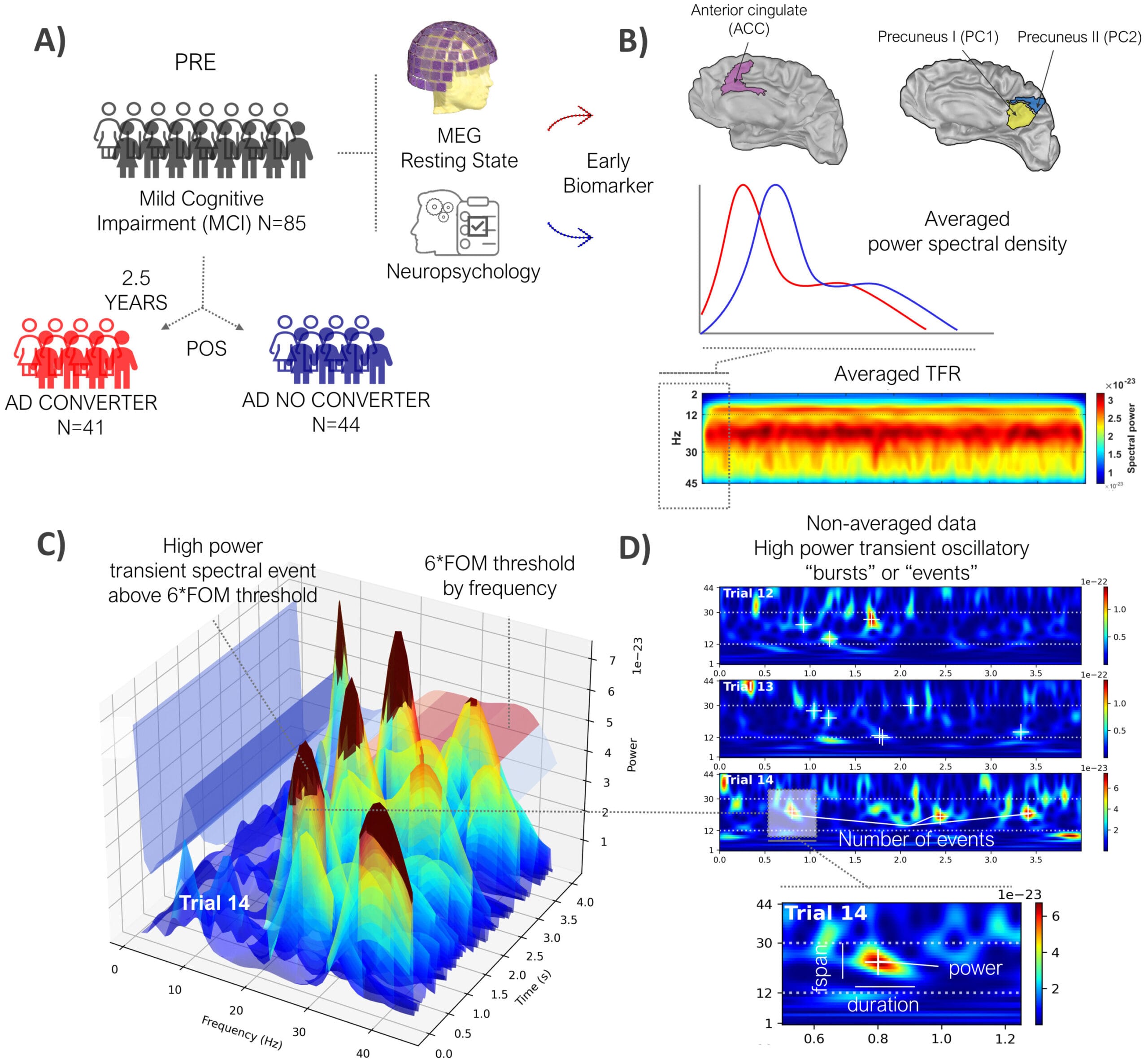
The study tracked 85 individuals who had been diagnosed with mild cognitive impairment (MCI), a condition that often, but not always, precedes Alzheimer’s. Over the course of several years, the researchers monitored these patients, looking for signs of cognitive decline. But instead of focusing on traditional memory tests or genetic risk factors, they turned to the brain’s own electrical activity for answers.
Using magnetoencephalography, or MEG—a technique that maps brain activity by detecting tiny magnetic fields generated by neurons—the team recorded the electrical rhythms of the participants’ brains while they were at rest, eyes closed, with no tasks or stimuli. This state, often considered “neutral,” actually reveals profound insights into brain function.
But there was a challenge: most existing analysis methods for MEG recordings reduce the complexity of the data, averaging it in ways that obscure critical signals. To overcome this, Jones and her colleagues turned to a tool they had developed themselves: the Spectral Events Toolbox, a sophisticated computational method that dissects neural activity into individual, measurable events.
Introducing the Spectral Events Toolbox
The Spectral Events Toolbox is more than just a filter—it’s a microscope for the mind. It breaks brain signals down into distinct bursts of activity, measuring when each event happens, how long it lasts, how powerful it is, and how frequently it occurs.
It’s been cited in over 300 scientific studies for its unique ability to reveal the rhythm of the brain, moment by moment. For this study, the researchers focused on events in the beta frequency band, which is associated with memory and cognitive processing—two key domains that Alzheimer’s disease severely disrupts.
“We focused on beta because it’s involved in memory functions, and disruptions in this frequency range have already been linked to Alzheimer’s pathology,” said Jones.
A Predictive Signal Hidden in Beta Waves
Using the Spectral Events Toolbox, the team examined beta-frequency activity in each patient’s brain and made a remarkable discovery. In patients who later developed Alzheimer’s disease, beta events were different—not in a dramatic way, but in subtle, consistent patterns.
“Two and a half years prior to their Alzheimer’s disease diagnosis, patients were producing beta events at a lower rate, shorter in duration and at a weaker power,” said Danylyna Shpakivska, the study’s lead author and a researcher at the Complutense University of Madrid, a collaborating institution.
This finding suggests that the brain begins to “go quiet” in specific frequency bands long before obvious cognitive symptoms arise. Importantly, this weakening signal seems to directly reflect how brain cells are functioning—not just how they’re structured or how much toxic protein they hold.
“To our knowledge, this is the first time scientists have looked at beta events in relation to Alzheimer’s disease,” Shpakivska added.
A New Kind of Biomarker for Alzheimer’s
For decades, researchers have searched for biomarkers—measurable biological indicators—that can diagnose Alzheimer’s before it reaches an advanced stage. So far, the most promising have been based on cerebrospinal fluid or blood, looking for telltale proteins like beta-amyloid and tau, which accumulate abnormally in the brains of Alzheimer’s patients.
But those protein-based biomarkers, while powerful, are still indirect. They reveal that something is going wrong, but not necessarily how the brain is responding.
“What we’re seeing with beta events is a functional biomarker,” said David Zhou, a postdoctoral researcher in Jones’ lab who is leading the next phase of the project. “We’re seeing how neurons are reacting to toxic stress in real time—how their communication is being affected, even before cognitive abilities are clearly impaired.”
This brain-based marker offers something new: the ability to measure function—not just structure or chemical buildup—and to do so noninvasively.
Toward Early Detection and Intervention
Alzheimer’s disease is notoriously difficult to detect early. Most people are diagnosed only after they begin to lose memory or struggle with everyday tasks. By then, damage to brain cells may be extensive and irreversible.
Jones believes the new findings could change that.
“The signal we’ve discovered can aid early detection,” she said. “Once our finding is replicated, clinicians could use our toolkit for early diagnosis and also to check whether their interventions are working.”
The Spectral Events Toolbox could one day be used during routine brain scans, flagging patients who may be silently progressing toward Alzheimer’s—even if they still appear cognitively intact on traditional tests.
This kind of early detection opens the door to early treatment, possibly before significant brain damage occurs. It also provides a valuable tool for testing new drugs, allowing researchers to see in real time whether a therapy is restoring healthy brain rhythms.
Modeling the Malfunction: What’s Going Wrong?
The next step, according to Jones, is to understand why these beta events are changing.
“Now that we’ve uncovered beta event features that predict Alzheimer’s disease progression, our next step is to study the mechanisms of generation using computational neural modeling tools,” she explained.
By building computer models that mimic brain networks, the team hopes to reconstruct how toxic proteins and inflammation disrupt neural communication—and how that disruption manifests as weaker beta events.
“If we can recreate what’s going wrong in the brain to generate that signal, then we can work with our collaborators to test therapeutics that might be able to correct the problem,” Jones said.
Hope on the Horizon
Alzheimer’s disease affects more than 55 million people worldwide, and that number is expected to double in the coming decades. It slowly erodes memory, personality, and identity—not only devastating individuals but placing immense strain on families and healthcare systems.
But studies like this one bring a new kind of hope—rooted not just in technology or discovery, but in understanding.
“Detecting Alzheimer’s earlier gives us more time—time to plan, time to intervene, time to hope,” said Zhou.
With a simple resting-state brain scan, and the right computational lens, researchers are learning how to hear the early whispers of the disease long before it roars. Those whispers may one day become warning signs—and, ultimately, lifesaving clues.
More information: Danylyna Shpakivska-Bilan et al, High-power transient 12–30 Hz beta event features as early biomarkers of Alzheimer’s disease conversion: An MEG study, Imaging Neuroscience (2025). DOI: 10.1162/IMAG.a.69