In a compelling new study that could redefine the future of antiviral medicine, scientists at the Cleveland Clinic have discovered a crucial biochemical switch in the body’s immune system—a modification to a protein called MDA5 that may determine how effectively we detect and respond to viral threats.
Published in the prestigious Proceedings of the National Academy of Sciences, the research zooms in on how this single protein modification—a process known as ISGylation—supercharges the immune system’s ability to recognize viral intruders and mount a defensive counterattack. Without it, the immune response falters, and viruses can run rampant, leading to severe complications like virus-induced heart inflammation.
It’s a breakthrough not just for understanding how our bodies fend off illness, but also for crafting future antiviral therapies that could work across a broad spectrum of viruses—something science has long pursued, but rarely achieved.
The Immune System’s Early Warning System
At the heart of this discovery is MDA5, a sentry protein in our cells that’s always on the lookout for signs of viral invasion. Part of the innate immune system—our body’s first, fast-acting defense—MDA5 detects double-stranded RNA, a hallmark of many viruses, including coronaviruses, enteroviruses, and mosquito-borne pathogens.
But MDA5 doesn’t act alone. After the body produces this protein, it needs fine-tuning—like installing upgrades in a freshly assembled robot. That’s where protein modifications come in.
These modifications are small but mighty. Enzymes attach chemical tags to specific locations on proteins, changing how they function. One such tag, ISG15, is at the center of this discovery. When it latches onto MDA5 through a process called ISGylation, it flips the immune system into high gear.
The Experiment: Disarming the Defense
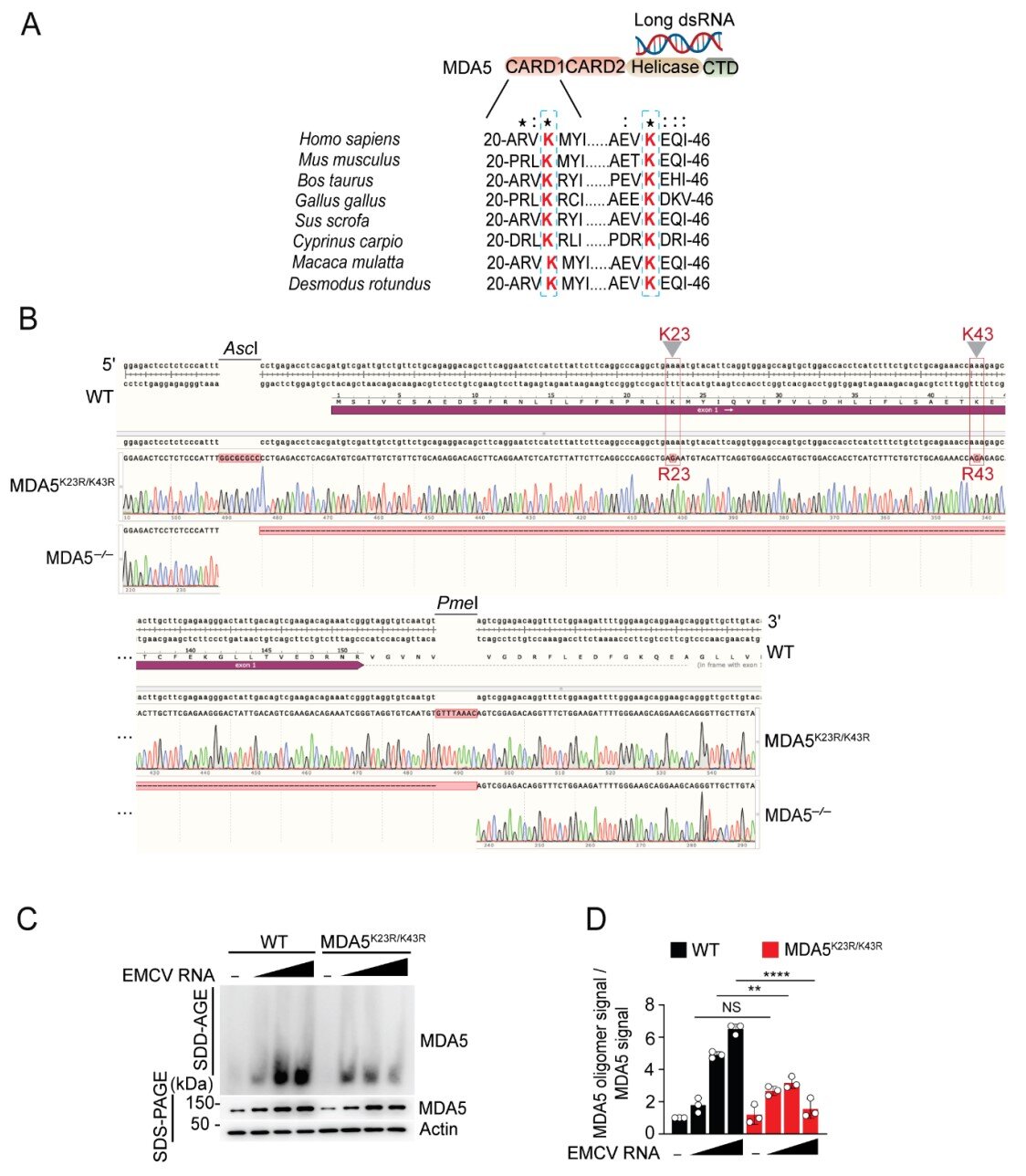
To test just how important ISGylation is for immune defense, Dr. Michaela Gack and her team created a unique model with help from the Case Western Reserve Transgenic and Targeting Facility. They genetically engineered a version of MDA5 stripped of the sites where ISG15 would normally attach.
The results were striking. Removing ISGylation was nearly equivalent to erasing MDA5 entirely. The immune system’s ability to detect the encephalomyocarditis virus (EMCV) plummeted. Infections worsened dramatically. Viral replication surged. Heart inflammation became more severe.
“We saw that without this one modification, MDA5 was practically useless,” explained Dr. Lucky Sarkar, the study’s lead author and a postdoctoral researcher in Dr. Gack’s lab. “That tells us just how essential ISGylation is—not only to MDA5’s activity but to the entire innate immune response.”
A Broader View: Beyond Just One Virus
What makes this finding even more exciting is its relevance beyond EMCV. Previous research from Dr. Gack’s team showed that MDA5 is responsible for sensing a wide range of viruses—coronaviruses like SARS-CoV-2, mosquito-transmitted viruses like Chikungunya, and more.
The ISGylation mechanism may be the master key that unlocks immunity across diverse virus families. In other words, it could become the foundation for broad-spectrum antivirals—a concept that has eluded medicine for decades.
“Most antiviral drugs and vaccines are custom-made for individual viruses,” Dr. Gack noted. “That’s incredibly effective for known pathogens, but it’s useless when something new emerges—just look at how COVID-19 caught the world off guard.”
But what if instead of targeting the virus, we could boost the host—amplifying the immune system’s universal sensors like MDA5 to fight back against anything that enters the body?
That’s exactly what this research opens the door to.
The Power of Protein Modifications
To understand why this is such a big deal, let’s rewind briefly to the cellular level.
When your cells detect a virus, they don’t just release an alarm—they undergo an internal transformation. Proteins are reprogrammed through modifications like ISGylation, phosphorylation, or ubiquitination. These changes equip proteins with new abilities—activating them, altering their location, or even marking them for destruction.
Think of it like preparing soldiers for battle. The base protein is the recruit, but protein modifications are the weapons, armor, and orders.
MDA5 is one of the body’s elite soldiers, and ISGylation is its most vital piece of armor. Without it, the soldier might as well be walking into battle unarmed.
From Lab Bench to Bedside: What’s Next?
The findings have electrified the immunology and virology communities, not just because of their elegance, but because of their translational potential.
“This is step one toward something revolutionary,” Dr. Gack said. “If we can figure out how to manipulate ISGylation or enhance it in the right cells at the right time, we could develop antiviral treatments that work across the board—even for viruses we haven’t discovered yet.”
Imagine a world where the next pandemic doesn’t require months or years of scrambling to develop a vaccine. Instead, doctors could stimulate a patient’s own ISGylation process, supercharging MDA5 and similar proteins to fight off the threat immediately.
Of course, there’s a lot more research needed before this vision becomes a reality. Scientists still need to map out the enzymes involved in the ISGylation pathway, discover how to control them safely, and determine which cells are the best targets for this kind of intervention.
But the promise is there—and growing.
The Bigger Picture: Why It Matters
Infectious diseases remain one of humanity’s greatest threats. From emerging zoonotic viruses to mutating strains of influenza and resurgent diseases like dengue and measles, the landscape of viral threats is constantly evolving.
Traditional antivirals and vaccines, while incredibly valuable, are often reactive tools. They’re designed after a virus strikes. Broad-spectrum antivirals represent a proactive shield—a way to defend ourselves against the unknown.
The work from the Gack Lab highlights an essential shift in how we think about immunity. Instead of endlessly chasing viruses, we can turn inward—enhancing our body’s natural defenses through molecular precision.
And at the center of it all is a tiny protein tag—ISG15—that might just be the key to future-proofing human health.
Final Thoughts
Science is often a story of small details unlocking grand possibilities. The discovery of MDA5 ISGylation’s role in viral detection is one such moment. It’s a molecular insight that could have monumental impact, not just for immunologists and virologists, but for all of us navigating a world where the next viral outbreak is not a matter of if, but when.
In this microscopic modification lies a macroscopic hope: a world better defended, not just by medicine, but by our own cells—smarter, faster, and stronger in the face of viral attack.
Stay tuned—because this could be the first chapter in a new era of immunity.
Reference: Lucky Sarkar et al, MDA5 ISGylation is crucial for immune signaling to control viral replication and pathogenesis, Proceedings of the National Academy of Sciences (2025). DOI: 10.1073/pnas.2420190122