Aging is, at its core, a story written in our DNA. As the years pass, the genetic code in our cells quietly collects more and more mutations. These small errors in our genetic blueprint are a natural byproduct of life — the result of chemical reactions, environmental stress, and the wear-and-tear of cellular machinery. Over decades, this accumulation can contribute to age-related diseases, organ decline, and the eventual breakdown of the body’s systems.
But a new study published in Science Advances has revealed an extraordinary exception to this rule. In the delicate, tightly protected genetic material of human egg cells, one crucial type of DNA damage seems to defy the march of time. Specifically, the mitochondrial DNA (mtDNA) inside a woman’s immature egg cells does not appear to accumulate mutations as she ages — a finding that challenges long-held assumptions about reproductive biology.
The Power Plants of the Cell
To understand why this discovery is so striking, it helps to know a little about mitochondria — the bean-shaped organelles often described as the “powerhouses” of the cell. Mitochondria convert nutrients into the chemical energy that fuels nearly every cellular process. Unlike other parts of the cell, mitochondria have their own DNA, which is separate from the DNA in the cell’s nucleus.
Mitochondrial DNA is passed down exclusively from mothers to their children, making it a direct genetic link from one generation to the next. While most mtDNA mutations are harmless, some can cause devastating disorders such as Leigh Syndrome, which in children can lead to seizures, developmental regression, and heart failure. Given their role in sustaining life, protecting mitochondria from harmful mutations is crucial — especially in the egg cells that will shape the health of future generations.
Putting Egg Cells Under the Microscope
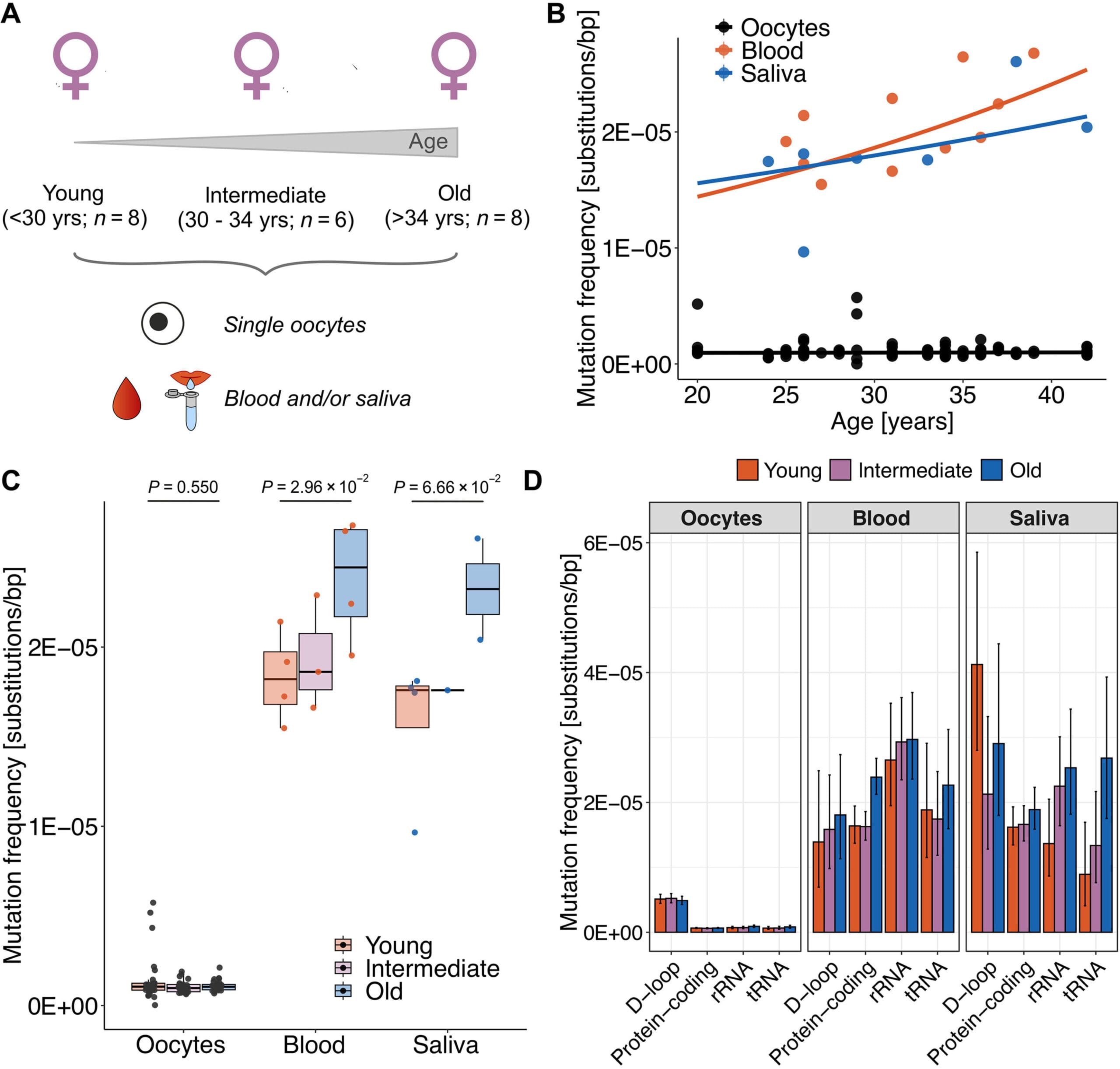
The new study examined 80 single oocytes — immature egg cells — collected from 22 women aged 20 to 42. Using advanced DNA-sequencing techniques, researchers carefully analyzed the mitochondrial DNA within each egg and compared it with mtDNA from the same women’s blood and saliva cells.
The results were striking: while the mtDNA in blood and saliva cells clearly accumulated more mutations with age, the mtDNA in egg cells showed no such increase. It was as if the oocytes had a built-in shield, protecting their mitochondrial DNA from the gradual genetic erosion seen elsewhere in the body.
“We found that mtDNA in human oocytes is protected against accumulation of mutations with aging and has functional consequences,” the authors wrote, suggesting this preservation might be a biological adaptation to ensure healthy reproduction — particularly relevant in a world where many women are choosing to have children later in life.
Strategic Protection in the Blueprint of Life
Interestingly, when mutations were present in egg cell mtDNA, they tended to occur in non-coding regions — stretches of DNA that do not directly make proteins. In the coding regions, where instructions for making vital mitochondrial proteins are stored, mutations were rare. This selective protection hints at a highly refined evolutionary safeguard: it’s not just about keeping the mtDNA intact, but about keeping the most important parts of it as pristine as possible.
Such precision in genetic maintenance makes sense when you consider the stakes. A single egg cell represents the potential start of an entirely new human life. Any damage in its mitochondrial DNA could be passed down to the child, possibly affecting health for decades to come. Protecting this DNA is, in essence, protecting the genetic future of humanity.
Reassurance for Older Mothers — With Caveats
The findings also carry a hopeful message for women who delay motherhood. While previous research has shown that older mothers face a higher risk of passing on chromosomal abnormalities — such as those linked to Down syndrome — it was long assumed that mitochondrial mutations followed the same pattern. This new study challenges that assumption, suggesting that maternal age might not have as much impact on mtDNA integrity as once thought.
That said, the researchers caution against over-interpreting the results. The study’s sample size was relatively small, and it did not track changes across a woman’s full reproductive lifespan. More research is needed to confirm whether these protective mechanisms remain equally effective in women well beyond the age range studied.
A Window Into Evolution’s Priorities
If confirmed by larger studies, this discovery could reshape our understanding of female fertility and aging. It suggests that the human body has evolved specialized strategies to keep egg cell mtDNA in near-pristine condition for decades, even as the rest of the body’s cells experience inevitable genetic wear.
Such protection makes evolutionary sense. A woman is born with all the egg cells she will ever have, and these cells may wait decades before being called into action. Preserving the mitochondrial DNA within them ensures that, when the time comes, they are still capable of producing healthy offspring.
The Next Steps
For now, the findings open a promising new chapter in reproductive biology. They could lead to improved fertility counseling, helping women make informed decisions about the timing of pregnancy. They might also inspire new avenues of research into how egg cells maintain their mitochondrial integrity — and whether this ability can be replicated in other cell types to slow aging or prevent disease.
What’s clear is that nature has hidden remarkable protective strategies in the most fundamental building blocks of life. As one researcher put it, the study “reminds us that biology doesn’t just let life happen — it actively works to safeguard it.”
More information: Barbara Arbeithuber et al, Allele frequency selection and no age-related increase in human oocyte mitochondrial mutations, Science Advances (2025). DOI: 10.1126/sciadv.adw4954