Dopamine is more than a chemical; it’s a storyteller, shaping the narrative of our daily lives. From the moment we open our eyes in the morning to the moments we feel joy, motivation, or focus, dopamine is there, guiding our thoughts, feelings, and actions. It is a small molecule derived from the amino acid tyrosine, but its impact on the brain is monumental. It plays a pivotal role in regulating movement, emotion, reward, and a wide array of cognitive functions, including attention and memory.
But dopamine’s influence doesn’t act like a flood. It’s more like a symphony—timed, modulated, and exquisitely precise. The human brain must keep dopamine levels in careful balance, dialing up or down its effects depending on the task at hand. When this dance is disrupted, the results can be devastating, manifesting in psychiatric and neurological disorders ranging from ADHD and schizophrenia to addiction and autism.
Now, a breakthrough study led by researchers from Florida Atlantic University (FAU) has uncovered a potential new way to recalibrate this delicate system. Their approach centers on a surprising target—not dopamine itself, but a receptor typically associated with opioid signaling. This discovery could pave the way for safer, more precise treatments for brain disorders where dopamine signaling has gone awry.
The Dopamine Transporter: Nano-Vacuum or Nano-Faucet?
To understand how this new treatment works, we first need to delve into the secret life of dopamine in the brain. When dopamine is released from a neuron into the synapse—the tiny gap between nerve cells—it quickly binds to receptors on neighboring neurons to transmit a signal. But this signal must be short-lived; otherwise, it would overwhelm the system. That’s where a protein called the dopamine transporter (DAT) comes into play.
DAT functions like a microscopic vacuum cleaner, rapidly sucking dopamine back into the neuron to stop the signal. It’s an elegant system, keeping dopamine’s message crisp and controlled.
But what happens when that vacuum malfunctions?
In some individuals, a rare genetic mutation known as DAT Val559 causes DAT to run in reverse. Rather than clearing dopamine from the synapse, the mutant transporter leaks it out, like a faulty faucet. This may sound like a recipe for heightened pleasure or even addiction, given dopamine’s reputation as the “reward” chemical. But the truth is more complicated.
As Randy D. Blakely, Ph.D., senior author of the FAU study, explains, this slow, steady leak dulls the brain’s ability to detect meaningful changes in dopamine levels. “Unlike the sharp rise in dopamine that triggers euphoria and can lead to addiction,” says Blakely, “dopamine that dribbles out through a leaky transporter makes it hard for a receptive brain cell to detect the normal rapid rise in dopamine that acts to fine-tune our thinking and emotions.”
In other words, this leak doesn’t make the brain hyper-rewarded—it makes it confused.
Dopamine and the Roots of Neuropsychiatric Disorders
This confusion can have serious consequences. The DAT Val559 mutation has been associated with a host of psychiatric diagnoses, including attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and bipolar disorder (BPD). Individuals with this mutation often struggle with inattention, mood instability, and compulsive behaviors.
Traditional treatments for these conditions often target dopamine more broadly. Stimulants like amphetamines, for instance, flood the brain with dopamine to improve focus and reduce hyperactivity. While effective for many, these medications can come with serious side effects, including increased risk of addiction.
So how can we fine-tune dopamine signaling without overloading the brain’s reward circuits?
That’s the question Blakely and his team set out to answer. And their answer lies in an unlikely place: the opioid system.
The Kappa Opioid Receptor: An Unexpected Ally
Opioids are best known for their powerful pain-relieving and euphoric effects. Endogenous opioids—those made by the body—modulate pain, stress, and mood. Illicit opioids like heroin and fentanyl hijack this system to produce intense pleasure, often with devastating consequences.
But the opioid system is not monolithic. It comprises several different receptor types, including the well-known mu opioid receptor (MOR) and the lesser-known kappa opioid receptor (KOR). Unlike MOR, which increases dopamine release and is associated with pleasure, KOR activation suppresses dopamine signaling. It does this by reducing dopamine release and increasing the number of transporters that clean up dopamine from the synapse.
In their study, published in Molecular Psychiatry, Blakely and collaborators took an unconventional approach: instead of activating KOR, they blocked it using a long-acting drug known as a KOR antagonist. The goal was to prevent the transporter from reaching the cell surface, where it leaks dopamine into the synapse.
Their hypothesis was bold: if KOR antagonists could stop the leaky transporter from doing damage, they might normalize dopamine signaling and restore healthy brain function.
And that’s exactly what happened.
Repairing the Leak: Mouse Models and Behavioral Rescue
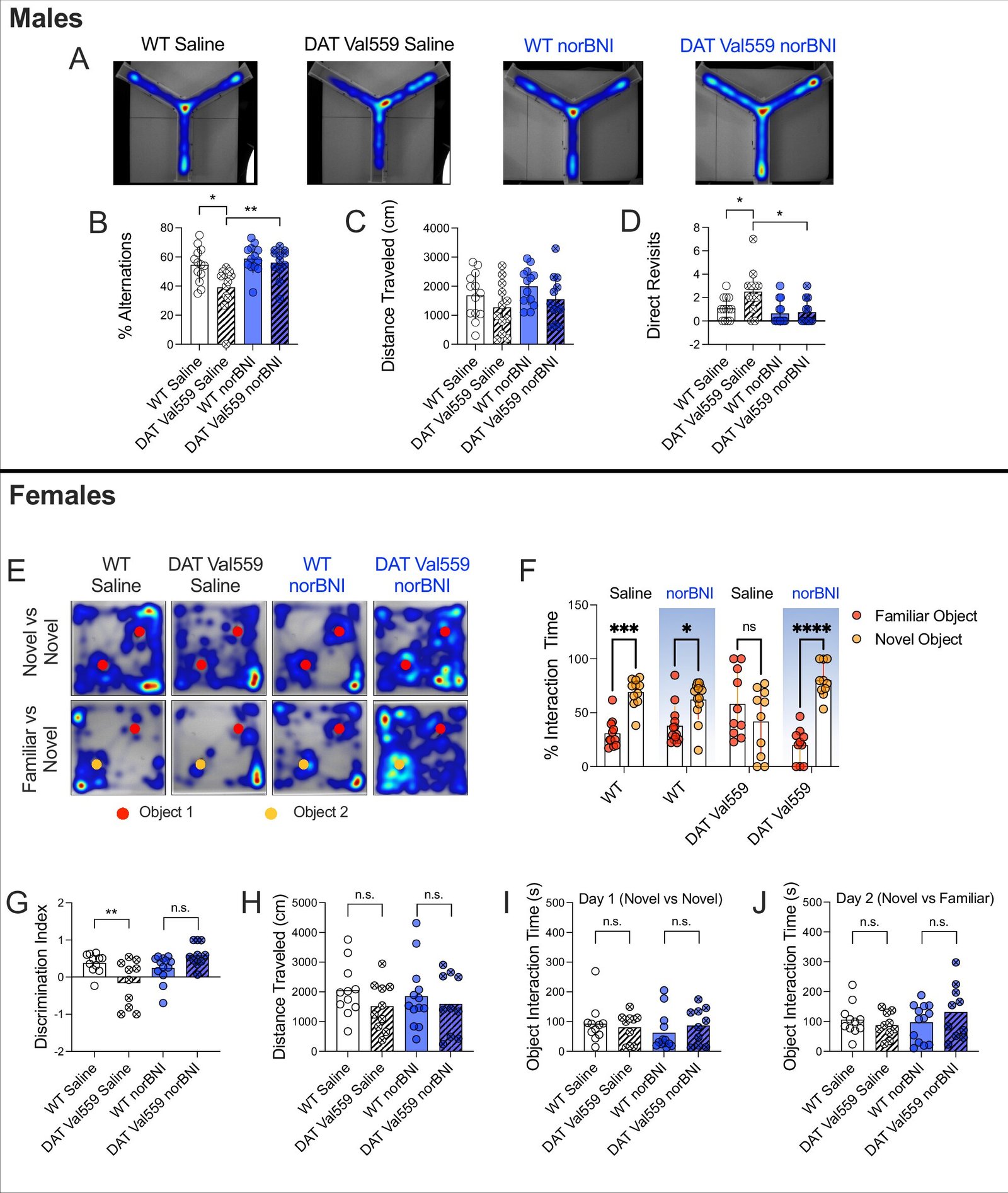
The researchers tested their hypothesis using genetically engineered mice that carry the DAT Val559 mutation. These mice exhibit behavioral traits that mirror aspects of human disorders, including hyperactivity, altered responses to psychostimulants, and problems with attention and social interaction. Remarkably, the effects of the mutation vary depending on the sex of the animal—a finding that mirrors emerging evidence in human psychiatric disorders.
When the researchers treated these mutant mice with a KOR-blocking drug, they observed a striking reversal of abnormal behaviors. The mutant DAT protein was less prevalent on the cell surface, reducing dopamine leakage and restoring more normal signaling dynamics.
“We were able to correct multiple behavioral deficits seen in the mutant mice,” says Felix P. Mayer, Ph.D., first author of the study and now a faculty member at the University of Copenhagen. “Blocking KOR helped restore the brain’s ability to detect and respond to dopamine changes in real time.”
Equally important, the KOR antagonist had no discernible effect on mice with normal DAT. This suggests a key advantage of the approach: precision. Unlike broad-spectrum dopamine boosters, KOR blockers may offer benefits specifically to those whose dopamine systems are misregulated, without the widespread side effects associated with current treatments.
Precision Psychiatry and the Promise of Personalized Medicine
At first glance, a treatment targeting a rare mutation might seem limited in scope. But Blakely believes the implications could extend far beyond DAT Val559. “We and others have found that DAT proteins are sensitively regulated by multiple pathways inside cells,” he says. “Changes in any one of these pathways—whether due to genetics, environment, or developmental timing—could produce behavioral disorders that would benefit from KOR blockers.”
Indeed, the broader concept emerging from this work is one of precision psychiatry: treatments tailored not to broad diagnostic categories, but to the specific molecular dysfunctions underlying each person’s condition. By understanding how proteins like DAT are regulated, scientists can identify new intervention points—and avoid the blunt-force tools of the past.
As Blakely notes, “The discovery of genetic variation in proteins that tightly regulate brain dopamine signaling, no matter how rare, can still offer insights into more common disease mechanisms.”
A Path Forward: From Mice to Humans
The leap from mouse models to human treatments is always a significant one. Still, the study lays a strong foundation for future work. The safety profile of KOR antagonists, combined with their apparent selectivity for pathological states, makes them attractive candidates for clinical trials.
Crucially, the effects of KOR antagonism were not limited by sex. Although the DAT Val559 mutation caused different behavioral patterns in male and female mice, blocking KOR improved symptoms in both groups. This points to the potential for developing sex-inclusive therapies—an important step forward in a field that has often neglected biological sex as a variable in research.
The next steps will involve testing whether KOR antagonists can provide similar benefits in other models of dopamine dysregulation, including those without the DAT Val559 mutation. If so, the implications for conditions like ADHD, ASD, and bipolar disorder could be profound.
The Future of Dopamine Research
Dopamine remains one of neuroscience’s most fascinating and enigmatic molecules. It is both the spark of euphoria and the quiet driver of focus. It governs some of our most basic behaviors—seeking, learning, remembering—but can also contribute to our most painful dysfunctions.
The FAU team’s discovery offers not just a new treatment approach, but a new way of thinking about how dopamine is regulated in the brain. It reveals how molecules from one signaling system—the opioid system—can be leveraged to fine-tune another. It underscores the importance of understanding not just the quantity of a neurotransmitter, but its rhythm, timing, and context.
And above all, it reinforces a central truth of modern neuroscience: small molecules can have huge consequences. By understanding their movements, their pathways, and their interactions, we move closer to solving the mysteries of the mind—and to offering relief to those whose brains have lost their balance.
Reference: Felix P. Mayer et al, Kappa opioid receptor antagonism restores phosphorylation, trafficking and behavior induced by a disease-associated dopamine transporter variant, Molecular Psychiatry (2025). DOI: 10.1038/s41380-025-03055-4