Inside every human cell, a delicate symphony of molecular interactions governs whether a cell lives, divides, or dies. It’s a choreography choreographed over billions of years of evolution—orderly, balanced, and tightly regulated. But sometimes, one of the dancers breaks free, defying the script and setting off a chain reaction that leads to cancer. In a groundbreaking study published in Science, a team of researchers led by Dr. Marc Therrien at Université de Montréal has unveiled exactly how this molecular rebellion takes place—and, remarkably, how it might be reversed.
At the heart of this story is a protein called BRAF, a crucial molecular switch embedded in one of the cell’s most important communication lines: the MAPK pathway. This signaling highway determines how cells respond to external cues—whether to grow, differentiate, or stay put. When functioning properly, the pathway keeps our cells in harmony. But when BRAF goes rogue, it can hijack this system, sending uncontrolled “grow!” signals that drive cancers of the skin, thyroid, colon, and lungs.
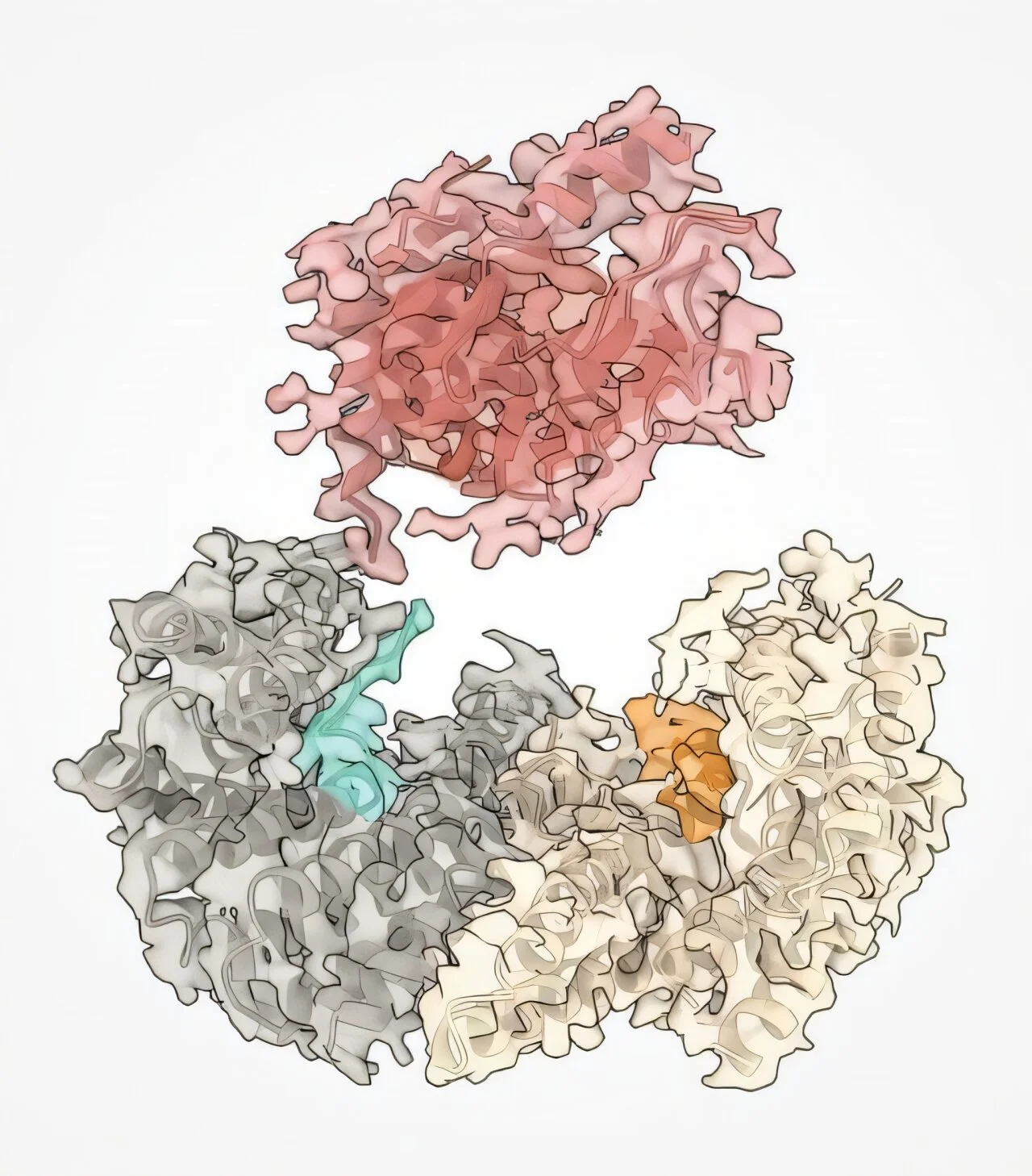
Now, using high-resolution cryo-electron microscopy, Therrien’s team has caught this saboteur in the act—watching in atomic detail as mutated BRAF proteins slip past the cell’s safety checks by masquerading as their activated form. Even more astonishing, the researchers have found a way to strip the disguise and restore the protein to its dormant, healthy shape using small molecule drugs. This is not just a scientific first—it’s a major leap toward a new generation of cancer therapies.
The Molecular Switchboard of Life—and Death
To understand how such a seemingly small change can wreak such havoc, we need to explore the MAPK pathway, short for Mitogen-Activated Protein Kinase. This intracellular network processes signals that originate outside the cell—such as growth factors or hormones—and translates them into specific instructions inside the nucleus.
BRAF is one of the key nodes in this pathway. In its dormant form, the protein is held in check by a mechanism known as autoinhibition—a kind of molecular off-switch that prevents accidental activation. When the cell receives a legitimate signal to grow, autoinhibition is released, BRAF switches on, and the message is passed downstream to other proteins, eventually altering gene expression and triggering cell division.
But BRAF is also a prime target for oncogenic mutations—those that give rise to cancer. Mutations in the BRAF gene can disable its self-control mechanisms, leaving the protein locked in a permanently “on” state. The result? A torrent of growth signals with no off-ramp, like a fire alarm that never stops ringing.
Catching BRAF in Disguise
The new study, led by Therrien’s team at the Institute for Research in Immunology and Cancer (IRIC), reveals exactly how these mutations alter the protein’s structure in ways that mimic its naturally activated form. Using cryo-electron microscopy, which enables researchers to visualize molecular structures at near-atomic resolution, the team saw that mutant BRAF folds itself into a configuration nearly identical to the active version.
In this conformation, BRAF essentially “fools” the cell’s regulatory machinery. Its internal autoinhibitory lock no longer fits the altered key. The result is a protein that is not just uncontrolled—it’s undetectably uncontrolled.
It’s a molecular masquerade, and one that proves incredibly hard to interrupt. Previous cancer drugs targeting mutant BRAF often worked only temporarily, as cells found ways around the blockage or mutated further to regain activity. To develop more durable treatments, researchers needed to understand the structure in fine detail. Therrien’s team has now provided that crucial roadmap.
The Alpha-C Helix: A Tiny Structure with Big Consequences
Zooming in even further, the study zeroed in on a small but crucial segment of the BRAF protein called the alpha-C helix. This spiral-shaped structure acts as a mechanical lever within the protein, changing position during activation.
In mutant forms of BRAF, the alpha-C helix locks into the “on” position. It’s this twist—literally—that enables the protein to simulate an activated state, even when no external signal is present. Like a car engine stuck in drive, the mutated BRAF never idles.
Here, the researchers saw a tantalizing opportunity: If they could use small molecules to nudge this helix back into its autoinhibited state, they might be able to restore the protein’s normal behavior. And that’s exactly what they did.
Rewinding the Clock on Cancer-Driving Proteins
Using a series of experimental small molecule inhibitors, the team managed to reconfigure the mutant BRAF protein, restoring its dormant structure in the lab. It’s the biochemical equivalent of removing a disguise—or perhaps more accurately, defusing a bomb.
This is the first time scientists have succeeded in fully converting an oncogenic BRAF mutant back into its inactive form using small molecules. It marks a radical shift in strategy. Traditional cancer drugs often try to block the effects of mutant proteins; this approach seeks to normalize them.
This distinction is critical. Rather than simply silencing BRAF or killing the cell outright, these molecules offer the potential for molecular reprogramming—a more nuanced, potentially less toxic approach that targets the root of the problem rather than its symptoms.
Implications for the Future of Cancer Therapy
More than half of all cancers involve abnormalities in the MAPK signaling pathway, and many of these are driven by BRAF mutations. The most notorious among them—BRAF V600E—is common in melanoma and other aggressive cancers.
While current BRAF inhibitors like vemurafenib and dabrafenib have shown success, especially in melanomas, they often encounter resistance over time. Tumors adapt. Secondary mutations emerge. Cancer finds a way.
By contrast, the mechanism uncovered by Therrien and colleagues suggests a different paradigm—one that doesn’t just inhibit BRAF but coaxes it back to sanity. If the mutant protein can be converted into a more docile form, it may be possible to restore regulation to the pathway without fully shutting it down, avoiding some of the toxic side effects of blunt-force therapies.
Furthermore, the detailed structural insights from cryo-EM give drug developers a powerful blueprint. With the alpha-C helix now confirmed as a linchpin of oncogenic activation, pharmaceutical efforts can zero in on this feature to create next-generation allosteric inhibitors—drugs that reshape the protein rather than simply blocking its function.
A Global Collaboration Unraveling a Global Killer
This discovery didn’t emerge from one lab alone. The study was a collaborative effort, with co-first authors Hugo Lavoie, Ting Jin, and Driss Lajoie from IRIC working closely with teams at NYU Langone in New York, Université de Bordeaux in France, and the University of Calgary in Canada. Such international cooperation highlights how tackling cancer’s complexity demands diverse expertise and a global vision.
Together, these teams combined structural biology, molecular modeling, pharmacology, and cancer biology into a seamless investigative engine. The result is more than a scientific paper—it’s a landmark contribution to our understanding of how cancer hijacks normal cellular machinery, and how we might seize back control.
Toward a World Without Rogue Proteins
In the battle against cancer, researchers are not just looking for bigger weapons—they’re searching for smarter ones. Therrien’s work shows that the future of oncology may lie in precision tuning, not just carpet bombing. By understanding the exact contours of how proteins like BRAF go astray, we can begin to design treatments that don’t merely attack but recalibrate.
It’s an approach rooted in respect for the complexity of biology. After all, BRAF isn’t a foreign invader—it’s a native protein gone astray. The challenge is not just to destroy it, but to bring it back into balance.
And that, perhaps, is the most hopeful message of all: that even in cancer, the chaos can sometimes be reversed. That with enough insight, precision, and collaboration, the most dangerous molecular masquerades can be unmasked—and peace restored to the cellular stage.
Reference: Hugo Lavoie et al, BRAF oncogenic mutants evade autoinhibition through a common mechanism, Science (2025). DOI: 10.1126/science.adp2742