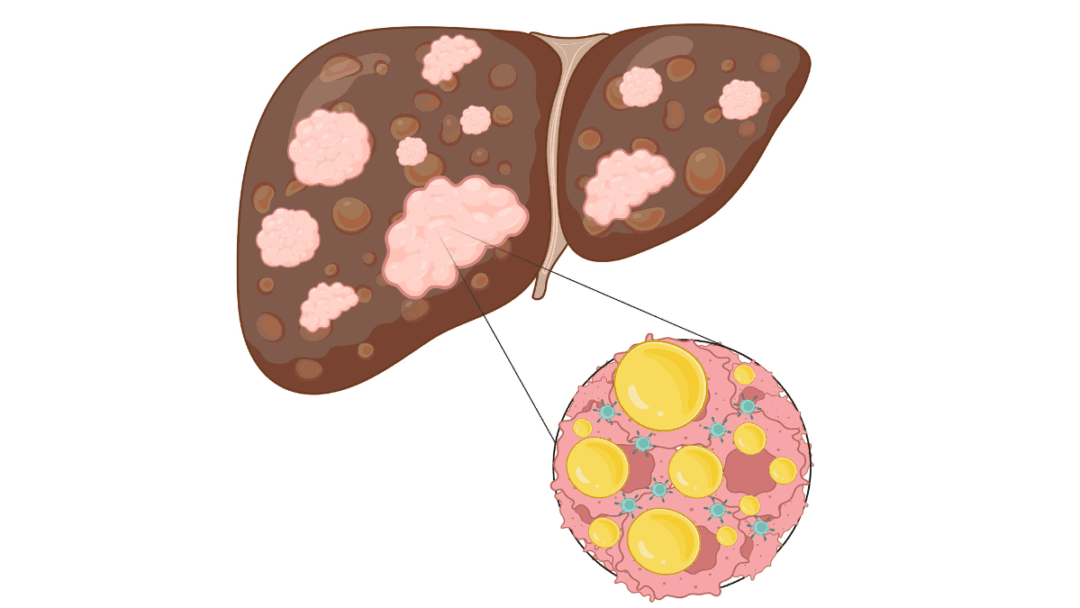
In a scientific development that could redefine the treatment of liver cancer, researchers from McMaster University, in collaboration with the biotech company Espervita Therapeutics, have discovered a way to harness the immune system by targeting how cancer cells use fat to grow. This breakthrough offers hope to the millions worldwide living with fatty liver disease—many of whom are unaware they’re at risk for developing liver cancer.
The study, recently published in the prestigious journal Nature, uncovers a novel treatment strategy: shut down a key enzyme that liver cancer cells depend on to process fat—and allow the immune system to do the rest. The results, while preliminary, are powerful: in animal models, this metabolic interference didn’t just slow down tumor growth, it allowed the body to recognize and destroy the cancer.
With fewer than 20% of liver cancer patients surviving beyond five years, this discovery represents a major shift in how scientists approach the disease.
When Fat Feeds the Fire
Liver cancer is notorious for its resistance to treatment and for developing silently, often emerging only once the disease has advanced. One of the primary risk factors is fatty liver disease—more formally known as metabolic dysfunction–associated steatotic liver disease (MASLD). It’s an increasingly common condition, tied to rising rates of obesity, poor diet, and sedentary lifestyles.
In Canada alone, MASLD affects more than eight million people. Roughly one in five of those individuals will develop metabolic dysfunction–associated steatohepatitis (MASH), a more severe form of the disease marked by inflammation and liver damage. Once this stage is reached, the risk of developing liver cancer increases dramatically.
What makes the situation even more alarming is that liver cancer cells thrive in this fatty environment. They adapt by altering their metabolism to feast on fat, growing faster and evading immune detection. For years, this metabolic link between fatty liver disease and cancer has been observed—but not fully understood. Until now.
Turning Off the Tumor’s Engine
Led by Professor Gregory Steinberg, co-director of McMaster’s Center for Metabolism, Obesity and Diabetes Research, the team focused on an enzyme called ATP citrate lyase (ACLY). This enzyme acts like a metabolic switch, converting sugar into fat within cells—a process cancer cells heavily exploit.
“We designed a drug that selectively inhibits ACLY in the liver,” Steinberg explained. “By turning off this enzyme, we essentially starve the tumor of the fat it needs to grow. But what we didn’t expect was that this would also wake up the immune system.”
The drug, named EVT0185, was administered to mice suffering from MASH and liver cancer. In these mice, tumor growth slowed significantly—and something extraordinary happened. Immune cells, previously dormant or ineffective, began to attack the cancer.
A Surprise Twist: The B Cell Breakthrough
In cancer immunology, T cells have long been the celebrated warriors—famous for their role in detecting and destroying tumor cells. But in this study, the spotlight shifted to a lesser-known hero: B cells.
“Our findings were surprising,” said Jaya Gautam, lead author and research associate in McMaster’s Department of Medicine. “The immune response wasn’t led by the T cells we typically expect in cancer treatment—it was B cells that were activated. This is one of the first demonstrations that a drug targeting tumor metabolism can recruit B cells to fight cancer.”
B cells are usually known for producing antibodies, but their role in cancer immunity has remained somewhat mysterious. This research suggests that when cancer’s metabolic engine is disrupted, B cells may rush in to clear the battlefield—perhaps even more effectively than T cells in certain contexts.
A Metabolic Doorway to Better Treatment
The implications of this discovery are immense. For one, targeting tumor metabolism is a radically different approach from conventional chemotherapy or even immunotherapy. Instead of attacking cancer cells directly, this method alters the environment they rely on to grow—making them weaker, slower, and more visible to the immune system.
“This is one of the first studies to show that targeting metabolism in tumors can enable the immune system to kill liver cancer cells,” said Steinberg. “It opens the door to more effective prevention and treatment strategies for this deadly disease.”
This approach could also help overcome one of the biggest limitations in current cancer immunotherapy: the inability of the immune system to detect tumors in the first place. If cutting off fat metabolism reveals the cancer’s hiding place, it might improve outcomes not just for liver cancer, but for other fat-dependent cancers as well.
Looking Ahead: From Mice to Humans
While the results in mice are promising, researchers are cautious about drawing conclusions too early. More studies will be needed to understand how ACLY inhibition enhances immune activity—and whether similar effects occur in human patients.
One major question is whether B cells will play the same starring role in people. Immunology is complex, and what happens in animal models doesn’t always translate directly to human biology. Still, the discovery opens new lines of inquiry and therapeutic design.
Clinical trials are the next step. If EVT0185 or similar drugs can be shown to work in humans, it could lead to a new class of liver cancer treatments—ones that combine metabolic intervention with immune system support.
Hope in a Field of Shadows
Liver cancer has long been a diagnosis of limited options and bleak outcomes. Its link to fatty liver disease has added urgency, especially as lifestyle-related illnesses surge globally. But this study shines a rare light through that darkness.
By uncovering a metabolic vulnerability in liver cancer—and showing that the immune system can be rallied to exploit it—researchers have given the world more than just a potential drug. They’ve given a new framework for thinking about cancer.
Where fat once fed the flames of cancer growth, it might now become the very target that weakens the tumor and empowers the body to fight back.
The war against liver cancer is far from over, but a new strategy has entered the field—one that enlists not just chemistry, but biology, metabolism, and the hidden powers of the immune system.
And for millions living with fatty liver disease, that could make all the difference.
More information: Gregory Steinberg, ACLY inhibition enhances tumour immunogenicity and resolves MASH-driven HCC, Nature (2025). DOI: 10.1038/s41586-025-09297-0. www.nature.com/articles/s41586-025-09297-0