When the liver is hurt, it tries to heal. But sometimes, in its effort to repair itself, it ends up doing more harm than good.
In a groundbreaking study, researchers at Toho University have unveiled a hidden cellular conversation that helps explain how this process goes awry—and how it could be stopped. Published in iScience, the new research reveals how certain cells inside the liver “talk” to each other in a way that spreads fibrosis, the dangerous buildup of scar tissue that can eventually lead to cirrhosis or liver cancer.
The discovery centers on two key molecular messengers—FGF18, a growth factor, and osteopontin (OPN), a protein known to promote fibrosis—and the surprising way they interact with liver cells. The findings paint a vivid picture of a self-reinforcing network of signals that silently fuels disease, one cell at a time.
When Healing Turns Harmful
Liver fibrosis begins as a defense mechanism. In chronic conditions like hepatitis or metabolic dysfunction-associated steatohepatitis (MASH), repeated injury to liver tissue causes certain cells to jump into action. The key players are hepatic stellate cells—star-shaped cells that, in healthy livers, quietly store vitamin A. But when the liver is under stress, they transform.
“They essentially flip a switch,” explains Dr. Takao Seki, Assistant Professor at Toho University’s Faculty of Medicine and lead author of the study. “They become myofibroblasts—cells that actively produce collagen and other structural proteins. It’s the body’s attempt to heal. But too much of this leads to scarring.”
Until now, scientists knew that activated stellate cells played a role in fibrosis. What was less clear was how that activation spread—and why fibrosis tends to grow even after the initial injury subsides.
This new research provides an answer: fibrosis spreads because the activated stellate cells send signals that awaken neighboring quiescent (inactive) stellate cells, effectively pulling them into the cycle of damage.
A Chain Reaction in the Liver
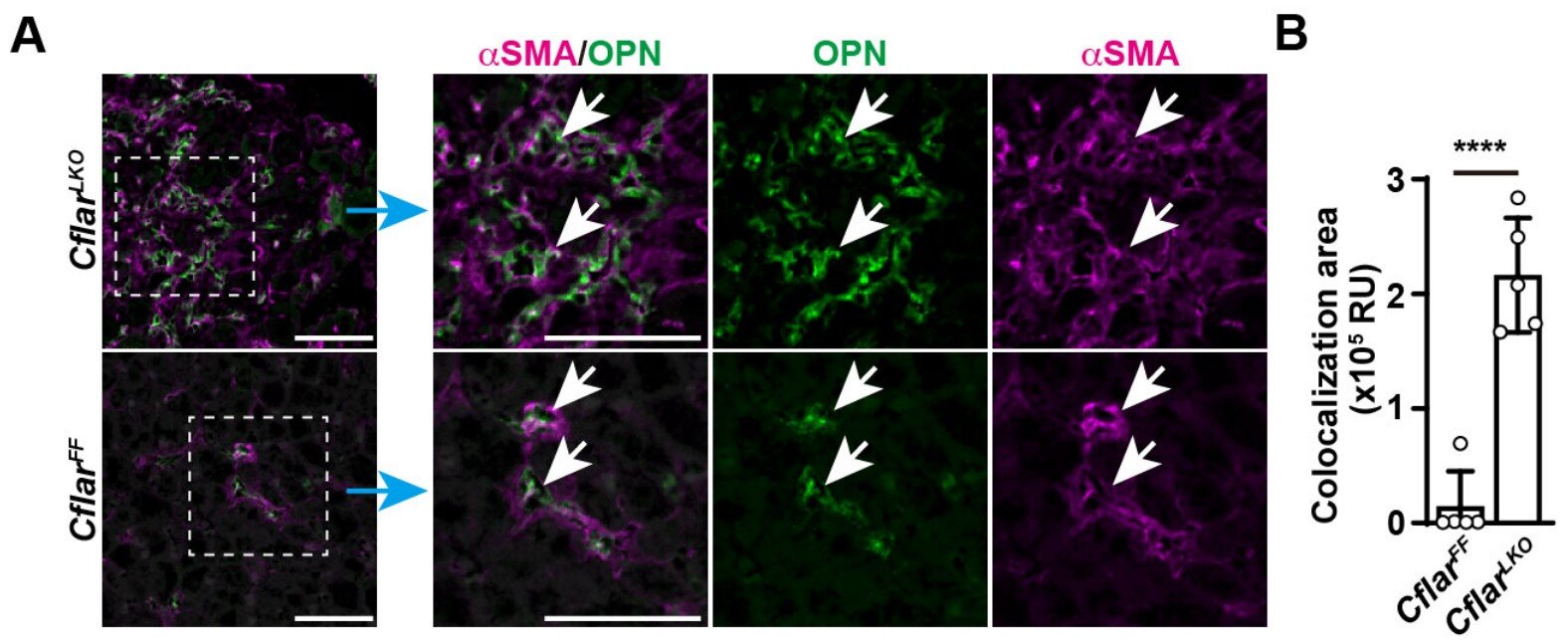
To uncover this process, the research team conducted a series of carefully controlled experiments. They found that when activated stellate cells are exposed to the growth factor FGF18, they begin producing large amounts of OPN—a protein previously known to be associated with fibrotic tissue.
But what came next was the real surprise.
OPN, it turns out, does not affect already active cells. Instead, it targets nearby quiescent stellate cells—those still in their resting, vitamin-A-storing state. Once hit with OPN, those quiet cells wake up and become active contributors to fibrosis. In other words, one active cell creates more.
“It’s a positive feedback loop,” said Dr. Hiroyasu Nakano, Specially Appointed Professor at Toho University and co-lead on the study. “And that loop doesn’t depend on ongoing injury from outside—it continues internally, from cell to cell.”
Using mouse models of liver fibrosis, the researchers confirmed the process in living organisms. They also pinpointed the receptor that allows OPN to exert its influence: a cell-surface protein called integrin. This protein acts like a molecular mailbox, receiving signals from OPN and passing them into the cell to initiate activation.
Why This Changes the Game
The discovery of the FGF18–OPN signaling axis offers a new lens through which to understand how liver fibrosis develops. Instead of a static accumulation of scar tissue, fibrosis now appears to be a dynamic, coordinated cellular response—driven by internal communication rather than external triggers alone.
“It’s a fundamental shift in how we view this process,” said Dr. Yuichi Tsuchiya, Associate Professor at the Faculty of Pharmaceutical Sciences and a collaborator on the study. “Fibrosis isn’t just a passive result of injury. It’s an active, self-propagating system.”
That insight is more than just scientifically interesting—it could also be lifesaving. Because FGF18 and OPN act selectively on stellate cells, they present an opportunity for highly targeted therapies. Instead of relying on broad-spectrum drugs that affect the entire liver (and often cause side effects), researchers might one day design treatments that specifically disrupt the FGF18–OPN conversation, stopping fibrosis in its tracks.
Toward a Future Without Cirrhosis
Liver fibrosis is a silent killer. In its early stages, it causes few symptoms. But as it progresses, the liver becomes increasingly stiff and scarred, reducing its ability to filter blood, regulate metabolism, and support overall health. Eventually, it can develop into cirrhosis—a condition that often ends in liver failure or cancer.
Current treatments for chronic liver disease focus mostly on managing symptoms or addressing the root cause, such as antiviral drugs for hepatitis. But there are few options to directly reverse or halt fibrosis itself.
That’s why the work led by Dr. Seki and Dr. Nakano is drawing so much attention.
“This study gives us a new blueprint,” said Dr. Minoru Tanaka, Division Chief at the National Center for Global Health and Medicine Research Institute and a key collaborator. “If we can interrupt this cell-to-cell activation cycle—by blocking FGF18 or neutralizing OPN—we may be able to prevent fibrosis from progressing, even in patients with ongoing liver injury.”
Cells That Whisper, Then Shout
In many ways, this research tells a larger story—one of how cells communicate, collaborate, and sometimes conspire to reshape the tissues they live in. The liver, long known for its resilience and regenerative power, is also vulnerable to the collective decisions made by its cellular inhabitants.
FGF18 and OPN are not just molecules. They are messengers, part of a complex biological language. When that language turns toxic, the results can be devastating. But by learning to read and rewrite these messages, scientists may finally be able to help the liver heal on its own terms.
“It’s like eavesdropping on a conversation we didn’t know was happening,” Dr. Nakano said. “And now that we can hear it, we have a chance to intervene.”
For millions of people living with liver disease, that chance couldn’t come soon enough.
Reference: Takao Seki et al, Intercellular communication between hepatic stellate cells and myofibroblasts mediated by osteopontin and FGF18 promotes liver fibrosis, iScience (2025). DOI: 10.1016/j.isci.2025.112932