For centuries, microscopes have been our windows into the microscopic world, enabling groundbreaking discoveries across medicine, biology, and countless other fields. But as scientific questions grow ever more complex, so too must our tools for investigating the mysteries of life. Traditional microscopes, for all their remarkable achievements, have limitations that often require trade-offs between sensitivity, precision, and the ability to capture dynamic changes in living cells. But now, a team of researchers from the University of Tokyo has made a groundbreaking advancement in microscopy that promises to transform our understanding of cellular processes, with far-reaching implications for both scientific research and industrial applications.
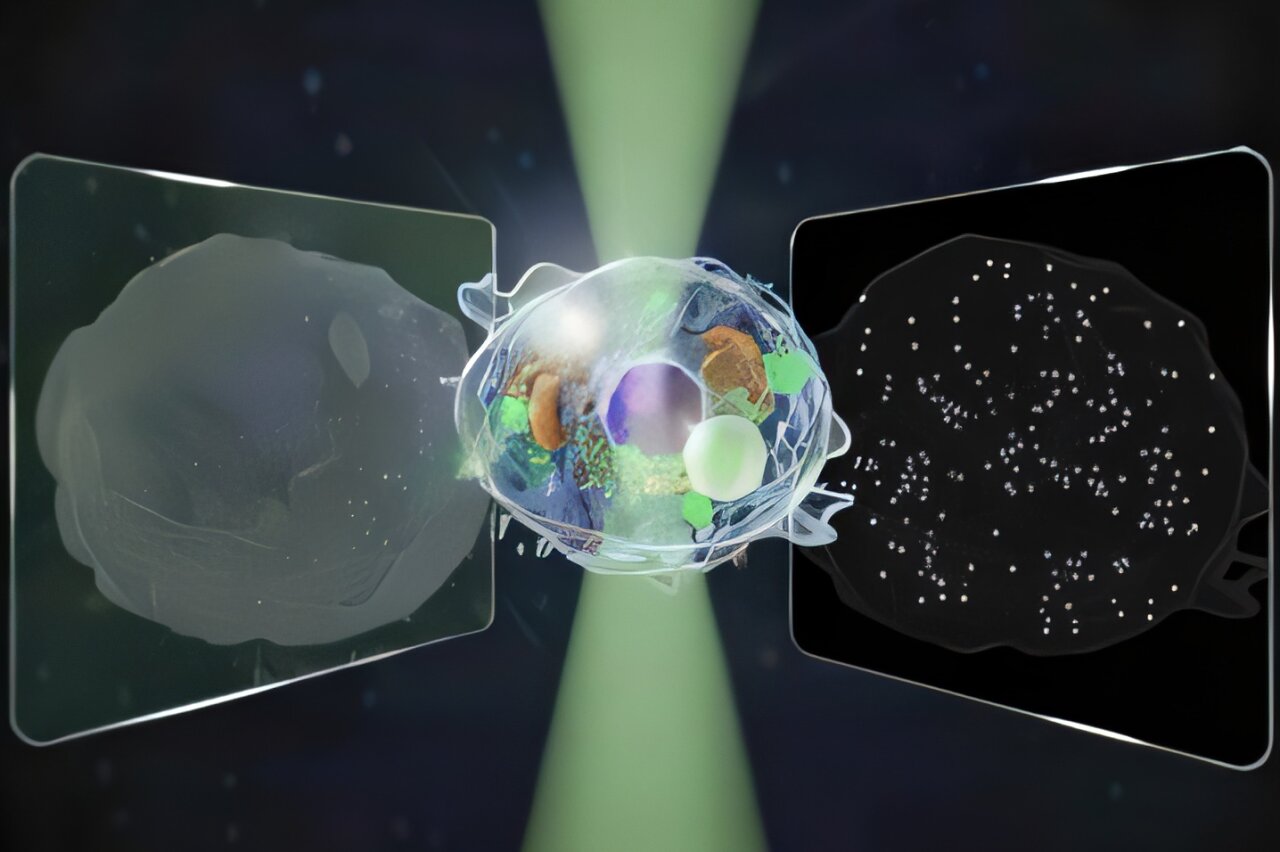
In a recent study published in Nature Communications, the team revealed a new type of microscope capable of detecting signals over an intensity range 14 times wider than conventional microscopes—without the need for additional dyes or labels. This “label-free” capability marks a significant leap forward in imaging technology, offering unprecedented insights into the dynamic processes of living cells while preserving their natural state.
The Challenge of Traditional Microscopy
Microscopes have long been essential tools for exploring the intricate structures of living organisms. The development of these instruments began as early as the 16th century, with pioneers like Antonie van Leeuwenhoek and Robert Hooke unlocking the first glimpses of the microbial world. Since then, microscopes have evolved to offer more detailed images and the ability to observe ever-smaller structures. But even as technological advancements have pushed the limits of magnification, traditional methods of imaging have always faced inherent challenges.
Most conventional microscopes rely on dyes and labels to highlight specific components within cells, allowing researchers to differentiate between different structures. However, these additional substances can interfere with the natural behavior of the cells, potentially distorting the very processes being observed. Furthermore, they can limit the ability to study dynamic changes over time, making long-term observations difficult. For instance, techniques like Quantitative Phase Microscopy (QPM) can provide excellent views of large cellular structures and give researchers a static snapshot of complex systems. But when it comes to tracking the movements of smaller particles or detecting structures at the nanoscale, QPM falls short.
On the other hand, Interferometric Scattering (iSCAT) microscopy is a technique that excels at detecting individual nanoparticles, such as proteins or viruses, and tracking their movement within the cell. However, this method only captures the tiniest of particles and lacks the broad view that techniques like QPM offer. This creates a trade-off: researchers could either have a detailed, static view of cellular structures or track the movement of single particles, but not both at the same time.
A New Approach to Imaging
Enter the University of Tokyo team, which sought to overcome this limitation. Led by researchers Kohki Horie, Keiichiro Toda, Takuma Nakamura, and Takuro Ideguchi, the team developed a new microscope that can simultaneously capture both forward-scattered and backward-scattered light. By measuring both types of light, the team could detect structures across a wide range of sizes, from the micro scale (over 100 nanometers) to the nano scale, without sacrificing either resolution or dynamic tracking.
The breakthrough approach was driven by a simple yet ambitious goal: to observe the internal dynamics of living cells in real time, using noninvasive methods. “I would like to understand dynamic processes inside living cells using noninvasive methods,” says Horie, one of the first authors of the study. The ability to simultaneously capture both forward and backward light allows for a broader and more detailed understanding of how cellular structures and individual particles interact and move within the cell.
To test their new method, the researchers turned their attention to a process that is both crucial and complex: cell death. The team sought to observe the motion of both cell structures and tiny particles during this critical biological process. By capturing light traveling in both directions, they were able to visualize the entire picture: the macroscopic changes in the cell’s structure as well as the nanoscopic movements of individual particles, all without the need for dyes or labels.
Overcoming the Challenges
One of the major challenges in developing this microscope was the need to cleanly separate the signals from forward and backward-scattered light, all while minimizing noise and interference. “Our biggest challenge,” says Toda, another lead author, “was cleanly separating two kinds of signals from a single image while keeping noise low and avoiding mixing between them.” Achieving this balance was critical to ensuring that the microscope could produce clear, accurate images of the dynamic processes within the cell.
The results were impressive. By comparing the forward and backward-scattered light, the team was able to not only track the motion of large cellular structures but also estimate the size and refractive index of tiny particles, such as proteins or other molecular structures. The refractive index is a critical property that describes how much light bends when passing through a material—an important characteristic for identifying and differentiating different types of particles.
This ability to measure both particle size and refractive index allows for much more precise and informative observations than previously possible. “We plan to study even smaller particles,” Toda adds, looking ahead to future research. “Such as exosomes and viruses, and to estimate their size and refractive index in different samples. We also want to reveal how living cells move toward death by controlling their state and double-checking our results with other techniques.”
Implications for Industry and Medicine
Beyond its scientific significance, this new microscope holds immense potential for practical applications in industries like pharmaceuticals and biotechnology. In particular, the ability to perform long-term, label-free imaging could significantly improve testing and quality control processes. For instance, pharmaceutical companies could use this technology to observe how drug molecules interact with cells, tracking the movement of both the drug and the cell structures it targets, without needing to add dyes or tags that might alter the process.
Similarly, biotechnology companies that rely on cell cultures could use the new microscope to observe dynamic cellular changes in real time, identifying how cells react to different conditions or substances over extended periods. This noninvasive imaging method could also be applied in diagnostics, where accurate and real-time monitoring of cellular activity is crucial for understanding disease processes, such as cancer or neurodegenerative conditions.
Moreover, the ability to study cellular processes like apoptosis (programmed cell death) with greater precision could lead to new insights into how diseases develop and progress. By understanding how cells die or transition between states, researchers could uncover novel therapeutic targets for a wide range of diseases, including cancer, Alzheimer’s disease, and other degenerative disorders.
The Future of Microscopy: What’s Next?
While this new microscope represents a significant leap forward, the team at the University of Tokyo is already looking ahead. The next steps involve refining the technology to study even smaller particles and to gain a more detailed understanding of the biological processes that occur within cells. They also plan to combine this new imaging technique with other methods to cross-verify results and build an even more comprehensive picture of cellular activity.
“We want to continue expanding the frontiers of what we can observe with this microscope,” says Horie. “By applying it to a broader range of biological phenomena, we hope to uncover new insights into the molecular machinery that drives life itself.”
This research marks a pivotal moment in the field of microscopy—a moment where we can observe the dynamic, living world inside our cells with unprecedented clarity, accuracy, and sensitivity. With its ability to track both the macroscopic and microscopic aspects of cell behavior, this new tool opens up exciting possibilities for understanding the hidden world of life at a deeper level than ever before. The future of microscopy is bright, and with it, the future of biomedical research and clinical applications is set to be transformed.
More information: Bidirectional quantitative scattering microscopy, Nature Communications (2025). DOI: 10.1038/s41467-025-65570-w