In a quiet laboratory at Tufts University, something extraordinary is taking place—not in the realm of artificial intelligence or quantum computers, but inside the very cells that make us human.
Modern humans have walked the Earth for over 200,000 years, each one shaped by a biological blueprint that has rarely faltered. From a single cell, a human unfolds—organs, limbs, a nervous system—assembled with astonishing fidelity, again and again, generation after generation. But what if those same human cells could be coaxed into building something else entirely? Something unfamiliar, something functional—but still alive?
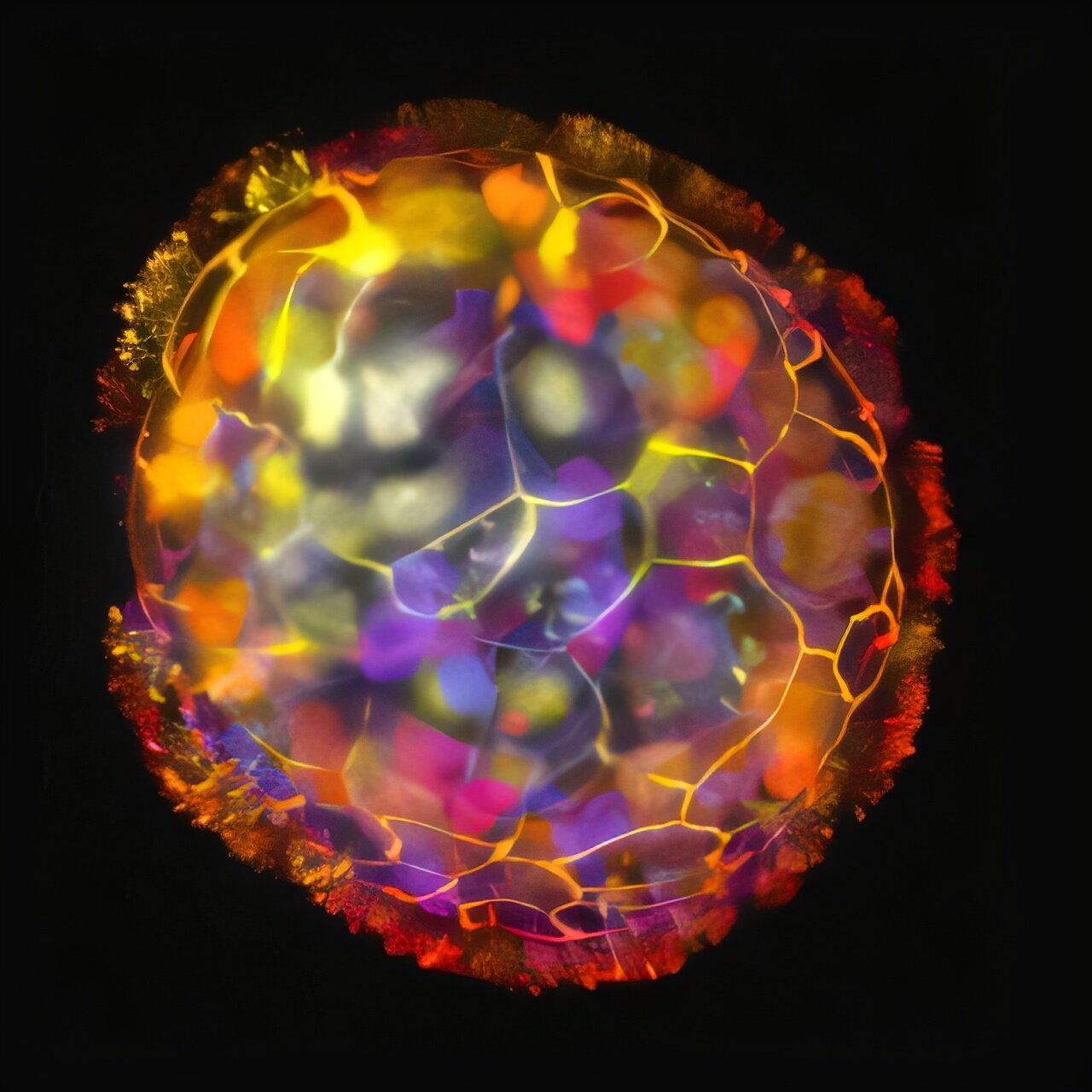
That question is at the heart of groundbreaking research led by biologist Michael Levin and scientist Gizem Gumuskaya at Tufts University. In a study recently published in Advanced Science, they unveil their latest creation: Anthrobots—tiny, living constructs made from human tracheal cells, capable of movement, self-repair, and even turning back their biological clocks.
More Than the Sum of Their Cells
The cells that make Anthrobots are not genetically altered. No CRISPR edits, no synthetic gene circuits. Instead, they are freed. Removed from their biological duties—no longer part of a lung, no longer tethered to the architecture of a human body—they begin to behave differently.
“Human cells are astonishingly adaptable,” says Levin, who holds the Vannevar Bush Professorship of Biology. “When released from their evolutionary obligations, they don’t just sit idle. They build.”
These Anthrobots come in a variety of forms—spherical, oblong, patchy with waving cilia that allow them to swim. Some can even “heal” when wounded. In one striking experiment, researchers sliced the bots with a scalpel. Rather than falling apart, the tiny creatures stitched themselves back together, returning to their original shape as though remembering what they were meant to be.
That memory—how cells recall shape—is not written in DNA alone. It arises from the interplay between genetic expression and a web of signals: electrical fields, mechanical stress, and chemical gradients. In Levin’s words, “Genes are the hardware. But the emergent instructions—the software—are shaped by the environment.”
The Return of Ancient Knowledge
When Gumuskaya, then a Ph.D. candidate, analyzed the gene expression inside Anthrobots, what she found was startling. More than 9,000 genes—almost half the human genome—were behaving differently than in their original adult state. The cells were activating programs seen not only in early human embryos but also in organisms from our distant evolutionary past.
“This wasn’t genetic manipulation,” she explains. “The cells did this on their own.”
Among the active genes were those responsible for embryonic processes like mesoderm-ectoderm transition—a critical step in forming internal tissues from the outer layers of an embryo. There were also genes guiding body axis formation: from head to tail (anterior-posterior) and back to belly (dorsal-ventral). Left-right patterning genes, however, didn’t activate—perhaps because the Anthrobots were mostly symmetrical spheres.
The finding hints at a cellular flexibility deeper than previously imagined. Despite being taken from a fully grown adult, these cells willingly dipped into the genetic memory of the womb—and before.
They remembered.
A Clock That Ticks in Reverse
One of the most astonishing findings from the Anthrobot experiments wasn’t just what the cells did, but how old they became.
To track the “biological age” of the cells, the researchers used epigenetic markers—molecular tags like methyl groups that accumulate over time, altering gene expression. These changes don’t affect the DNA sequence itself, but they influence how genes behave, and they correlate with the aging process.
One Anthrobot donor was a healthy 21-year-old. His cells, unsurprisingly, had an epigenetic age of 25—slightly ahead of his chronological age, as is common. But after transforming into Anthrobots, the cells’ biological age dropped to 18.7 years. That’s a 25% reduction—without any chemical rejuvenators, gene edits, or cloning.
Simply by reorganizing into a new living form, the cells had turned back time.
“I was stunned,” Gumuskaya recalls. “It suggests that shape alone—structure and interaction—can rewind the aging clock.”
Levin calls it the “age evidencing hypothesis.” If cells sense they’re participating in an embryonic-like process—building new structures, forming new patterns—they may begin acting younger, shedding the molecular wear-and-tear of adulthood.
“It’s as if the cells are trying to reconcile their function with their history,” he explains. “The logic of form is powerful enough to alter the biology of time.”
The Implications of Living Machines
Anthrobots are not science fiction. They are real, microscopic, and alive. They blur the line between organism and machine—simple enough to be assembled, yet complex enough to swim, self-heal, and rejuvenate.
And their potential uses reach far beyond the laboratory petri dish.
If these constructs can be made from a patient’s own cells, they could one day deliver drugs, clean up diseased tissues, or assist in wound repair—all without triggering an immune response. Their self-organizing nature suggests a future in which we design biological tools not through engineering blueprints, but by coaxing nature’s own processes into action.
And perhaps most significantly, the Anthrobots offer new clues into developmental diseases like birth defects. Understanding how cells spontaneously organize into structures—and why that process sometimes goes awry—could open new pathways for prevention and repair.
“Developmental biology is still filled with mysteries,” Levin says. “If we can decode the rules of self-assembly, we may not only grow new tissues but correct ones that have formed improperly.”
When Biology Becomes Design
At the heart of the Anthrobot project is a larger question: what is a body? If cells can build a creature that swims, heals, and ages in reverse—but isn’t a human, mouse, or frog—then perhaps “lifeform” is a more flexible concept than we once thought.
Anthrobots challenge our assumptions about identity. They are not clones. They are not machines. They are purpose-built collectives of living cells—more like a colony than a person, yet undeniably alive.
“We didn’t evolve to build Anthrobots,” Gumuskaya says. “But the capacity was always there, hidden in our cells, waiting to be unlocked.”
And it was unlocked not with engineering precision, but with a simple change in context. A tracheal cell removed from the body, placed in a novel environment, responds not by dying or freezing—but by building.
It adapts. It dreams a new form.
The Future of Embodied Intelligence
As we stand at the frontier of synthetic biology, the Anthrobot research raises profound philosophical questions. Are these constructs alive? Are they robots? Could they be conscious?
Not in any human sense, Levin emphasizes. But their ability to respond, adapt, and organize suggests they belong to a new category: embodied intelligence. Not intelligence that thinks, but intelligence that becomes.
In this view, cells are agents, not just parts. They compute. They solve problems. They “decide” to form structures based on local signals, shared history, and group behavior. They are more like ants in a colony than components in a machine.
“This is the new frontier,” Levin says. “We’re learning to speak the language of morphogenesis. It’s not just about genes—it’s about information, context, and collective behavior.”
Rewriting the Script of Life
Tufts’ work on Anthrobots isn’t just a biological oddity. It’s part of a larger reimagining of what life can be. If cells can build something new when given a new mission, then perhaps our own biology is far more malleable than we believed.
What if we could cue our cells to build what we need, when we need it? What if aging could be reversed not with drugs, but with shape? What if the distinction between natural and synthetic becomes irrelevant, and life becomes something we design—not with control, but with cooperation?
Anthrobots are a first step into that future. They are fragile, small, and mysterious. But they are also brimming with promise.
And somewhere, quietly swimming beneath a microscope, a cluster of once-forgotten cells has remembered how to be young again.
Reference: Gizem Gumuskaya et al, The Morphological, Behavioral, and Transcriptomic Life Cycle of Anthrobots, Advanced Science (2025). DOI: 10.1002/advs.202409330