Why do some people find it harder to connect, to bond, or to navigate the subtle dance of social interaction? For decades, autism research has searched for the hidden threads tying together genes, brain chemistry, and behavior. A team of researchers in Spain has now illuminated one of those threads, identifying for the first time how a mutation in the Shank3 gene—long associated with autism—alters the brain’s chemistry and disrupts social behaviors.
The discovery, published in Nature Communications, reveals that the key lies in a delicate brain hormone called vasopressin, a chemical messenger vital for regulating how we relate to one another. By uncovering exactly how this hormone is disrupted, the study not only clarifies a long-standing mystery but also points toward promising new therapies that could one day help people with autism improve social interaction without unwanted side effects.
The Role of Shank3: A Fragile Link in the Chain
The Shank3 gene has been known for years as a critical player in autism spectrum disorder. Mutations in this gene often result in difficulties with communication, sociability, and repetitive behaviors. But the biological pathway from this single genetic alteration to the rich complexity of social behavior has remained elusive—until now.
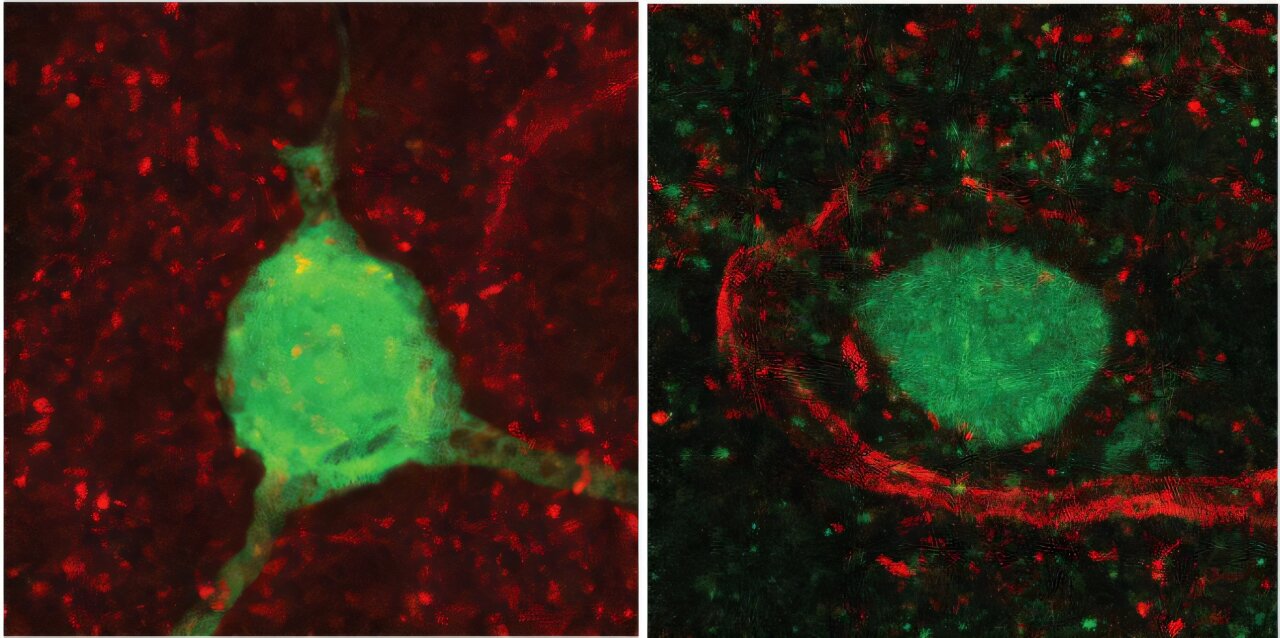
The team led by Félix Leroy at the Institute for Neurosciences (a joint center of Spain’s National Research Council and Miguel Hernández University) took on this challenge with a mouse model carrying the autism-associated Shank3 mutation. What they found was striking: the mutation disrupts a population of neurons responsible for releasing vasopressin, which in turn prevents the hormone from properly reaching a crucial brain region called the lateral septum.
This is no small glitch. The lateral septum is a hub where social behaviors are finely tuned. Without enough vasopressin reaching it, the mice became less sociable and lost the defensive aggression typically used to protect territory. The missing hormone, it turned out, was the missing key to unlocking the connection between genetics and behavior.
Vasopressin: The Messenger of Connection
Most people know vasopressin as a hormone that helps regulate water balance in the body, but in the brain, it plays a far more intimate role. It acts as a neurochemical bridge between individuals, regulating how social bonds form, how trust develops, and even how aggression is expressed in defense of territory or community.
In the mice studied, the Shank3 mutation led to a loss of vasopressin-producing neurons in a region called the bed nucleus of the stria terminalis (BNST). These neurons normally project to the lateral septum, releasing vasopressin precisely where it is needed to orchestrate social behaviors. With fewer neurons and less hormone reaching this target, the normal balance of sociability and aggression was disrupted.
This provided the first clear biological explanation for why Shank3 mutations cause social difficulties: the problem is not just in the genetic code, but in how that mutation cascades into neurochemical miscommunication.
Two Receptors, Two Pathways
What makes this discovery even more fascinating is how specific the mechanism turned out to be. The researchers found that vasopressin works through two distinct receptor pathways in the lateral septum:
- AVPR1a, which regulates sociability.
- AVPR1b, which governs social aggression.
By carefully manipulating these receptors, the scientists showed that it is possible to restore sociability without triggering aggression. This precision matters enormously. In theory, if a treatment simply boosted vasopressin levels everywhere in the brain, it could lead to unintended side effects, such as heightened aggression. But by identifying the exact receptor responsible for sociability, the researchers have opened the door to therapies that target only what is needed.
As Leroy explains, “We managed to improve sociability without increasing aggression, which is fundamental if we are thinking about a future treatment.”
A Technological Breakthrough in Real-Time
To make this discovery, the team used a groundbreaking tool: a vasopressin biosensor developed in collaboration with Yulong Li’s laboratory at Peking University. This biosensor allowed them to visualize, in real time, how vasopressin was being released in the brain.
For the first time, researchers could watch the hormone’s activity unfold, revealing not a broad dysfunction across the entire nervous system but a precise alteration in a specific brain circuit. This clarity was vital, narrowing the scope of potential therapies and deepening the understanding of how localized brain chemistry shapes complex social behaviors.
To ensure their findings held up, the team also partnered with the University of Zurich, where computational data analysis validated the reliability of the results.
Toward Personalized Therapies
The implications of this research go beyond mice. The results are protected under a patent application aimed at developing drugs that can selectively activate the AVPR1a receptor to improve sociability. Such drugs could, in the future, provide targeted treatments for people with autism who struggle with social interaction—without triggering side effects related to aggression.
Another intriguing layer of the findings involves sex differences. The study focused on male mice, in which the vasopressin pathway is more developed and territorial aggression is part of normal behavior. This choice was not arbitrary: autism is diagnosed more frequently in males, and the stronger involvement of vasopressin in their social circuitry could help explain why. Still, the researchers caution that autism may manifest differently in females, or even be underdiagnosed, suggesting that any future treatments will need to account for sex-specific differences.
“Our results suggest that future treatments could be personalized, taking these differences into account,” Leroy emphasizes.
The Bigger Picture: A New Era in Autism Research
This discovery is not just about one gene, one hormone, or one brain region. It is part of a broader movement in neuroscience to map the intricate circuitry that shapes human behavior. For years, autism research has identified dozens of genetic factors, but the path from DNA to lived experience has often remained murky. By showing exactly how a genetic mutation alters neurochemical signaling and, in turn, social behavior, this study sets a new standard for clarity.
The work is also part of the MotivatedBehaviors project, which explores how the lateral septum regulates social and motivated behaviors. In 2023, Leroy’s group published a study in Cell showing how another brain signal—corticotropin-releasing hormone—can suppress interaction with familiar individuals. Step by step, these discoveries are building a detailed map of the brain’s social machinery.
A Glimpse of Hope
For families and individuals living with autism, the journey is often one of unanswered questions and limited therapeutic options. While this research is still in the early stages and based on animal models, its message is profoundly hopeful. It suggests that the social difficulties tied to autism are not an unchangeable mystery, but a challenge that can be understood, mapped, and eventually addressed with precision therapies.
Science, at its best, is a light in the dark—a way of revealing the hidden mechanisms that shape our lives. In this case, the light falls on vasopressin, a tiny molecule that carries immense weight in how we connect with others. By tracing the pathway from Shank3 mutation to disrupted hormone release, the researchers have illuminated one of the brain’s most intimate secrets: the biological roots of human connection.
And in doing so, they have given us more than knowledge. They have given us the possibility of change.
More information: Maria Helena Bortolozzo-Gleich et al, Impaired vasopressin neuromodulation of the lateral septum leads to social behavior deficits in Shank3B+/- male mice, Nature Communications (2025). DOI: 10.1038/s41467-025-61994-6