The human brain is often described as the most complex structure in the universe, a network of billions of neurons weaving thoughts, emotions, and memories into the fabric of who we are. Yet, like any delicate system, it can be thrown into turmoil by forces meant to protect it. One such force is neuroinflammation, a prolonged activation of the brain’s immune system that, while essential in fighting infections, can sometimes tip the balance toward damage.
Neuroinflammation is not merely a byproduct of disease—it is increasingly recognized as a driver of many cognitive problems. When this hidden fire smolders for too long, it can impair mental function, disrupt memory, and contribute to neurodegenerative conditions like Alzheimer’s and Parkinson’s disease. Scientists around the world have long suspected that immune signaling within the brain plays a role in these declines, but until recently, the molecular “how” remained elusive.
Signals of Distress: Cytokines and the Brain
When the brain’s immune defenses are triggered, specialized cells release proteins known as cytokines. These molecules act as messengers, coordinating the immune response and shaping how different cells react to threats. Among them, one cytokine in particular—interleukin-1 (IL-1)—has drawn the attention of neuroscientists.
IL-1 is a powerful signal of alarm. While vital for orchestrating the body’s defense against infection, it also influences how neurons function. Some studies have suggested links between IL-1 and changes in memory, but the precise mechanisms were still shrouded in mystery. Could this inflammatory molecule really alter the way neurons encode and retrieve experiences?
The Parasite That Opened a Window
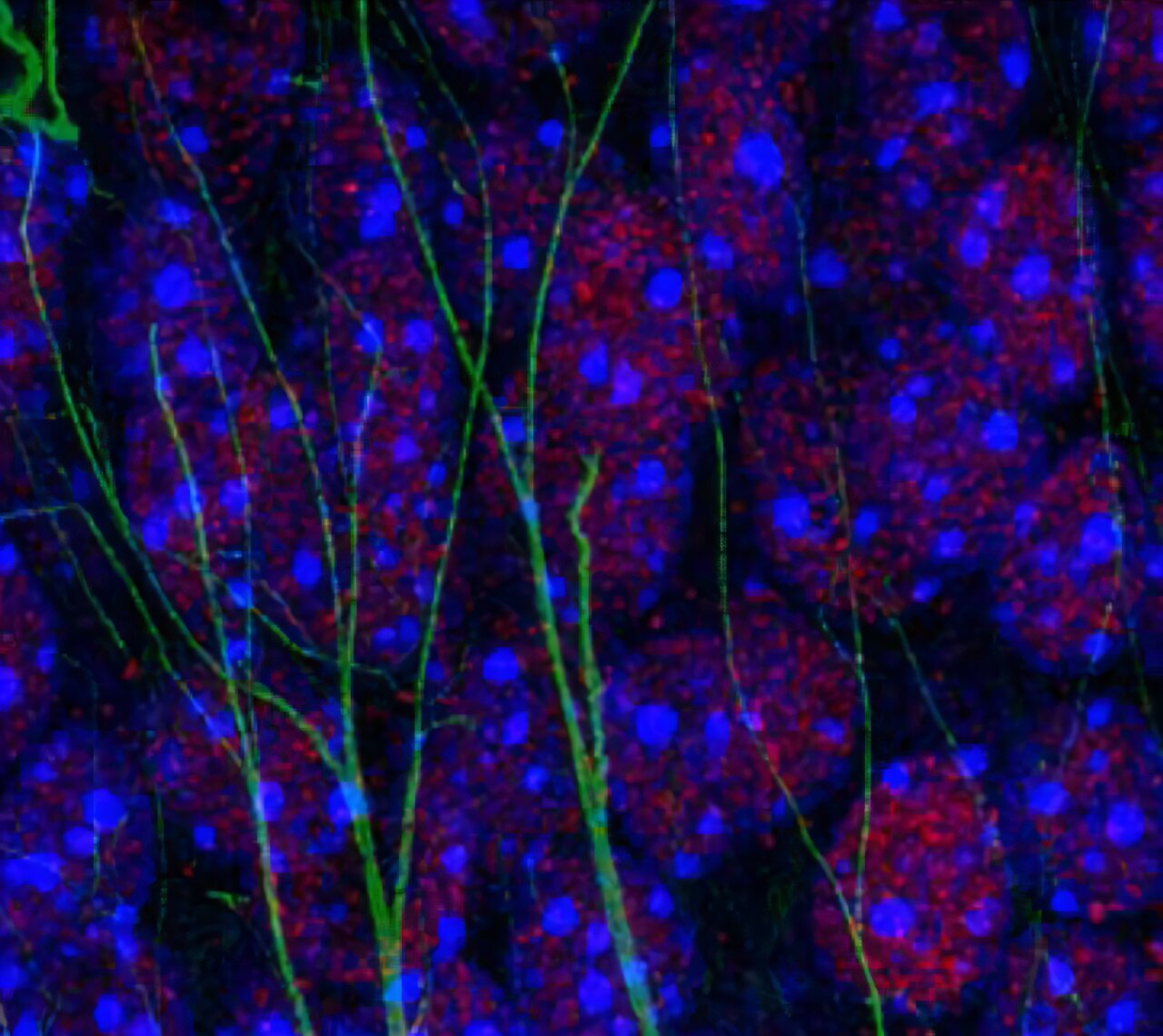
To explore this question, researchers at the University of Toulouse INSERM and CNRS turned to an unlikely culprit: Toxoplasma gondii (T. gondii). This microscopic parasite, infamous for causing toxoplasmosis, has a unique relationship with the brain. After infection, it can linger inside neurons for years, quietly fueling chronic neuroinflammation.
T. gondii is more common than many realize—around one-third of the human population has been exposed. Most infections remain silent, but in some cases, they leave behind subtle cognitive changes. This persistence makes the parasite an ideal model for studying how inflammation in the brain disrupts memory.
As Dr. Nicolas Blanchard, co-senior author of the study, explained: “T. gondii can chronically persist inside neurons of the brain, fueling neuroinflammation and cognitive problems. Our goal was not only to identify the inflammatory signals involved but to uncover the general molecular mechanisms linking neuroinflammation to neuronal dysfunction.”
Memory, DNA, and the Fragile Dance of Neurons
The study builds on earlier discoveries by Dr. Elsa Suberbielle, the other co-senior author. Her work revealed that spatial memory—our ability to remember places, routes, and contexts—relies on a surprising process within neurons: the controlled breaking and repairing of DNA.
This may sound alarming, but in fact, these temporary DNA breaks allow neurons to reorganize how genes are expressed, a process known as epigenetic regulation. Far from damaging the brain, this mechanism fine-tunes the way neurons respond to experience, especially in the hippocampus, the brain’s memory hub.
The problem arises when inflammation interferes. Could cytokines like IL-1 be disturbing this fragile dance of DNA repair, leaving memories fragmented and incomplete?
Two Models, One Answer
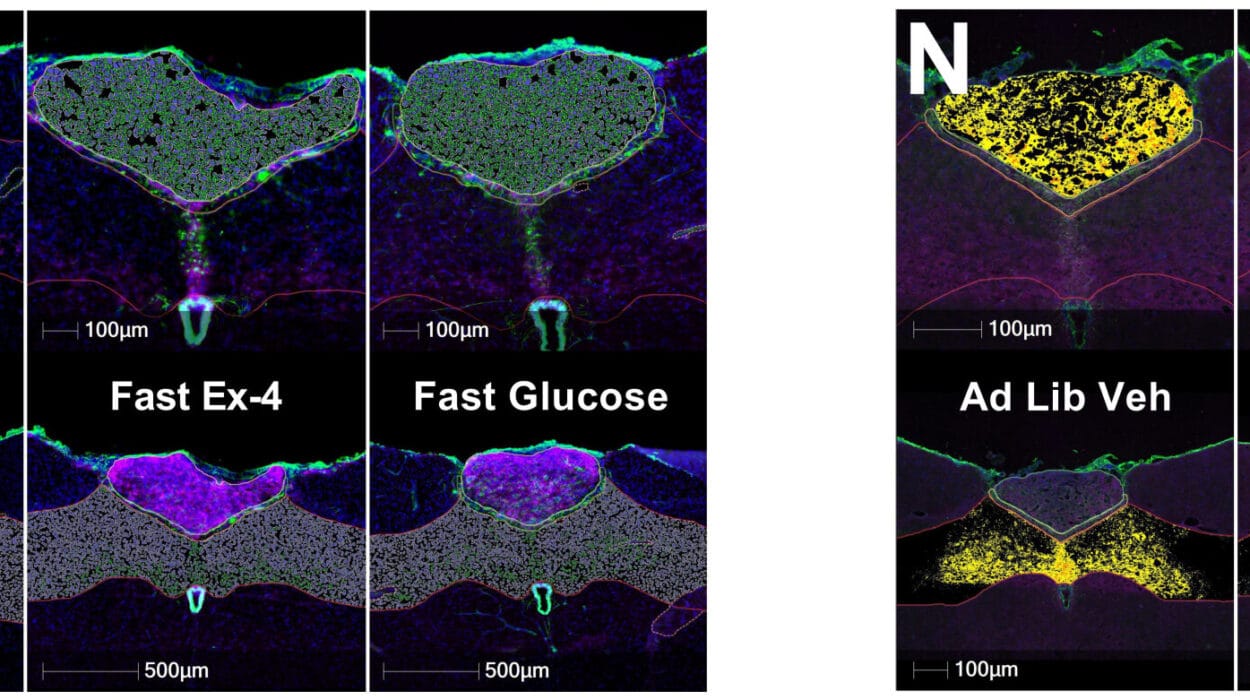
To test this, the researchers designed two powerful experiments. In one, mice were infected with T. gondii to mimic the chronic neuroinflammation seen in humans. In the other, the team artificially raised IL-1 levels in otherwise healthy mice, simulating the conditions of inflammatory diseases.
The results were striking. In both cases, the animals struggled with tasks requiring spatial memory. They failed to recall the location of objects they had previously explored and became disoriented in maze-like environments.
By blocking the ability of excitatory neurons in the hippocampus to recognize IL-1, the researchers uncovered the critical role of this cytokine. Without its receptor, neurons were shielded from the inflammatory storm, and the mice retained their ability to consolidate spatial memories.
“We showed that IL-1 alters the epigenetic regulation of neurons, providing the first molecular explanation of memory disturbances caused by this common parasite,” Blanchard explained.
A Glimmer of Hope
Perhaps the most exciting aspect of this research lies in its implications for treatment. The team found that interfering with IL-1 signaling—or protecting neurons from DNA break-related stress—could prevent memory impairments, even under high levels of brain inflammation.
This discovery suggests a new therapeutic pathway. If these findings hold true in humans, it may become possible to design drugs that shield memory-forming neurons from inflammatory damage. Such therapies could benefit not only those infected with T. gondii but also millions of people suffering from chronic inflammatory conditions, depression, and neurodegenerative diseases.
As Blanchard and Suberbielle put it: “Since interleukin-1 is elevated in many chronic inflammatory conditions, our study opens new avenues for treating memory-related deficits.”
Beyond Parasites: Toward a Universal Mechanism
Though the research began with a parasite, its reach extends much further. Chronic inflammation is a hallmark of many modern diseases, from viral infections like SARS-CoV-2 to metabolic disorders. The fact that IL-1 can alter memory at such a fundamental level points to a shared mechanism underlying diverse conditions.
This insight reframes how we think about brain health. Memory is not only a product of neurons firing together but also of the immune system’s balance within the brain. The immune system is not a distant defender but an intimate partner in shaping cognition.
The Human Dimension
The findings strike a deeply human chord. Memory is more than information storage—it is the essence of our personal identity, the thread that connects who we were yesterday with who we are today. To lose memory is to lose part of ourselves.
That such loss could stem not from an inevitable decay but from a potentially reversible inflammatory signal is profoundly hopeful. It suggests that, by understanding the dialogue between the immune system and neurons, we may one day protect the fragile narratives of our lives from being erased.
The Road Ahead
This work marks only the beginning. Translating discoveries from mice to humans is a complex challenge, requiring careful testing and refinement. Yet the principle is clear: neuroinflammation is not merely a background symptom—it is an active disruptor of cognition.
Future research will likely expand on these findings, exploring whether blocking IL-1 or related pathways can help people with Alzheimer’s, Parkinson’s, or even depression. Each step brings us closer to the possibility of safeguarding memory from the silent fire of inflammation.
Conclusion: When Science Meets Memory
The story of neuroinflammation is both scientific and profoundly personal. It shows us that the brain’s immune system, designed to protect us, can sometimes turn against our most cherished capacities. But it also reveals a path forward: with knowledge, we can transform vulnerability into resilience.
By uncovering the hidden links between cytokines, DNA repair, and memory, the researchers at Toulouse have illuminated a new frontier in neuroscience. Their work is a reminder that science is not only about molecules and cells—it is about preserving the stories that make us who we are.
In the end, the fight against neuroinflammation is not just about preventing disease. It is about protecting memory, identity, and the delicate spark of humanity that resides in every brain.
More information: Marcy Belloy et al, Toxoplasma gondii infection and chronic IL-1 elevation drive hippocampal DNA double-strand break signaling, leading to cognitive deficits, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-02041-x.