বিশ্বজুড়ে লক্ষ লক্ষ মানুষের জন্য, জ্বর কমে গেলে বা কাশি কমে গেলেও মহামারীটি সত্যিকার অর্থে শেষ হয়নি। কারও কারও জন্য, COVID-19 একটি অদৃশ্য ছায়া রেখে গেছে – লক্ষণগুলির একটি সমষ্টি যা অদৃশ্য হতে অস্বীকার করেছিল। লং COVID বা পোস্ট-COVID-19 সিন্ড্রোম নামে পরিচিত এই অবস্থা বিজ্ঞানীদের এবং ক্লান্ত রোগীদের উভয়কেই বিভ্রান্ত করেছে। মাধ্যাকর্ষণ শক্তির মতো অনুভব করা ক্লান্তি ভারী, শ্বাস যা কখনও পূর্ণ বলে মনে হয় না, “মস্তিষ্কের কুয়াশা” এর ধোঁয়ায় ভেসে যাওয়া চিন্তাভাবনা – ভাইরাসটি তাদের শরীর ছেড়ে যাওয়ার অনেক পরেও অগণিত মানুষের জন্য এগুলি বাস্তবতা।
But a striking pattern has emerged: women, far more than men, are disproportionately affected. Research published in Cell Reports Medicine has shed new light on this troubling disparity, uncovering biological clues that may finally explain why women experience longer, more severe symptoms of long COVID—and how we might begin to treat them.
When Recovery Never Comes
Long COVID is diagnosed when symptoms persist for three months or more after an acute SARS-CoV-2 infection. These symptoms can include neurological issues, respiratory difficulties, gastrointestinal problems, pain, depression, and an overwhelming exhaustion often resembling chronic fatigue syndrome.
According to Statistics Canada, as of June 2023, 3.5 million Canadians reported experiencing long COVID. Globally, the numbers are far higher—tens of millions of lives affected. The condition does not discriminate by age or health; many who were young, active, and only mildly ill with COVID-19 later found themselves trapped in a cycle of unrelenting fatigue and confusion.
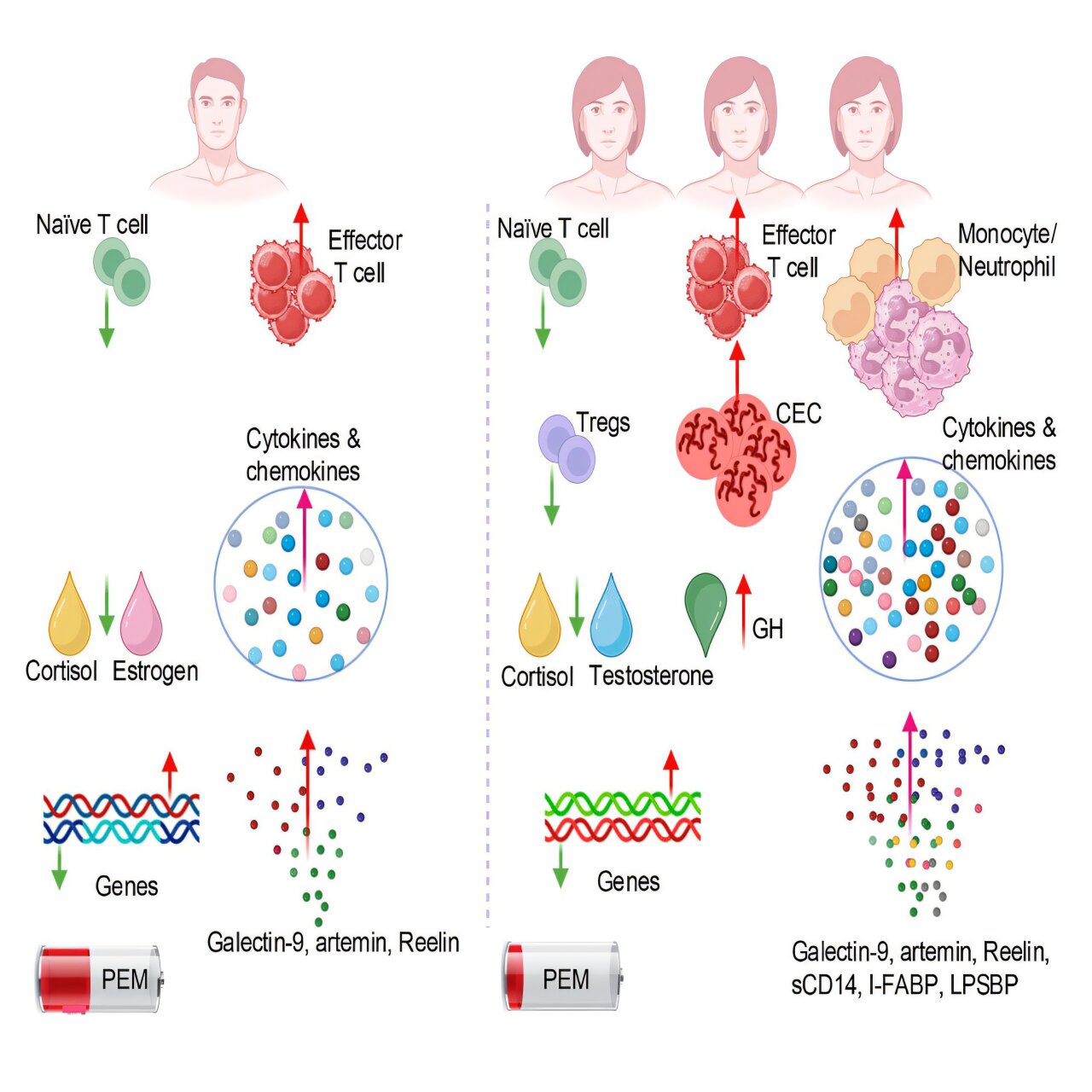
Yet among those afflicted, women are three times more likely than men to develop long COVID. Until now, this gap was a mystery. Why should a virus, which affects men more severely during acute infection, linger so stubbornly in women afterward?
The Study That Opened a Door
Immunologist Shokrollah Elahi and his team at the Mike Petryk School of Dentistry decided to look deeper. Their study, published in Cell Reports Medicine, analyzed blood and genetic samples from 78 long COVID patients one year after their initial infection. These were compared to 62 people who had recovered fully without lingering symptoms.
The researchers weren’t just looking for antibodies or leftover viral fragments—they were searching for patterns in immune cells, hormones, and molecular signals in the blood that could explain why the body refuses to reset. What they found was a distinct biological signature between women and men, suggesting that long COVID may not be one disease at all, but rather several overlapping syndromes influenced by sex-based immune responses.
The Leaky Gut Connection
One of the most striking discoveries was evidence of what scientists call “gut leakiness” in women with long COVID. This term refers to a condition where the lining of the intestines becomes inflamed or damaged, allowing toxins and bacterial components to seep into the bloodstream.
In the female patients, blood levels of certain biomarkers—such as intestinal fatty acid binding protein, lipopolysaccharide, and soluble CD14—were significantly elevated. These molecules are telltale signs of gut inflammation. Once they enter the bloodstream, they can trigger systemic inflammation throughout the body, affecting energy levels, mood, and even brain function.
Elahi explains that this vulnerability might begin at the earliest stages of infection. “There is a tendency that the females’ guts are more prone to viral infection,” he says. In other words, the virus may disrupt the gut barrier more easily in women, setting off a chain reaction that never fully resolves.
This ongoing gut inflammation could explain many of the hallmark symptoms of long COVID—chronic fatigue, digestive discomfort, and the feeling of being inflamed from the inside out.
Anemia and the Burden of Inflammation
Elahi’s team also found that women with long COVID showed signs of anemia, or reduced red blood cell production. Red blood cells are essential for transporting oxygen throughout the body, and when their levels drop, fatigue and cognitive impairment can follow.
The researchers believe this anemia is not due to iron deficiency, but to inflammatory suppression of blood production. Inflammation releases molecules that interfere with the bone marrow’s ability to make new blood cells. As Elahi notes, “Elevated inflammatory factors in females with long COVID adversely affect their blood production.”
Anemia adds another layer of exhaustion to a condition already characterized by deep fatigue. When your cells are starved of oxygen, even simple tasks can feel monumental.
The Hormonal Imbalance
Perhaps the most intriguing part of the study involved sex hormones. The team found that women with long COVID had lower testosterone levels, while male patients showed decreased estrogen, and both sexes had reduced cortisol, the hormone that helps regulate stress and inflammation.
For women, this drop in testosterone may be especially significant. Though often associated with men, testosterone plays important roles in the female body as well—particularly in controlling inflammation. The study found that women with lower testosterone levels had higher inflammation and more severe symptoms such as brain fog, pain, depression, and fatigue.
It’s a subtle but profound insight: hormonal balance may determine how resilient the body is to chronic inflammation after infection. The immune system and endocrine system are deeply intertwined, and when one falters, the other can spiral out of control.
A Mirror to Chronic Fatigue Syndrome
The pattern emerging from Elahi’s research bears a striking resemblance to another long-misunderstood illness: myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Like long COVID, ME/CFS disproportionately affects women and is marked by debilitating fatigue, brain fog, and immune dysfunction.
Both conditions involve chronic inflammation, hormonal irregularities, and immune system abnormalities. However, anemia—prominent in long COVID—has not been a hallmark of ME/CFS. This suggests that while the two syndromes may share pathways, long COVID might represent a broader or more complex version of post-viral fatigue.
The comparison also offers hope. Understanding one condition can illuminate the other, opening doors to treatments that might help both long COVID and ME/CFS patients.
A Global Puzzle Comes Into Focus
Elahi’s findings are supported by a recent international study published in the Journal of Clinical Investigation, which analyzed over 500 long COVID patients and identified anemia as a major biological factor. Across continents, research teams are converging on the same clues—pointing toward inflammation, blood abnormalities, and hormonal imbalance as key drivers of persistent post-COVID illness.
These discoveries don’t just explain what’s happening; they hint at how to heal. If inflammation and hormone disruption are central to long COVID, then therapies targeting these processes—such as anti-inflammatory drugs, hormone replacement, or treatments for anemia—could provide real relief.
Toward Personalized Treatment
Elahi envisions a future where long COVID treatment is tailored to the biology of each patient. “We need an individualized approach,” he says. Depending on test results, some may benefit from therapies that boost red blood cell production, others from targeted anti-inflammatories, and still others from carefully balanced hormone support.
His next step is to test these possibilities in animal models and, eventually, in human clinical trials. It’s a daunting task, but one with enormous potential. If successful, these treatments could transform not just long COVID care, but our understanding of how viral infections can trigger chronic disease.
The Human Cost Behind the Science
Behind every data point is a person who wakes up each morning still waiting to feel like themselves again. Many with long COVID find their lives transformed—careers interrupted, relationships strained, everyday tasks overwhelming. The study’s findings offer more than scientific insight; they offer validation.
For years, patients—especially women—have struggled to have their symptoms believed. The new evidence confirms what they have known in their bones: that their suffering is real, measurable, and rooted in biology.
More information: Integrated immune, hormonal, and transcriptomic profiling reveals sex-specific dysregulation in Long COVID patients with ME/CFS, Cell Reports Medicine (2025). DOI: 10.1016/j.xcrm.2025.102449. www.cell.com/cell-reports-medi … 2666-3791(25)00522-1
Gisela Gabernet et al, A multiomics recovery factor predicts long COVID in the IMPACC study, Journal of Clinical Investigation (2025). DOI: 10.1172/jci193698