In a groundbreaking revelation that could reshape biology textbooks and redefine medical strategies, scientists from The University of Manchester have challenged a fundamental concept about how cells divide—a process that has been taught to students for over a century. Published in the prestigious journal Science, this new research dissects the mechanics of cell division and rewrites the rules governing how cells generate diversity in living organisms.
For generations, students have learned that during mitosis, the parent cell adopts a spherical shape before dividing into two daughter cells of equal size and identical function. This symmetry in shape and role has been a cornerstone of cellular biology. However, the Manchester researchers, through advanced live imaging and molecular manipulation, have shown that this model is far too simplistic—and in many cases, entirely inaccurate.
The Cell that Defied Convention
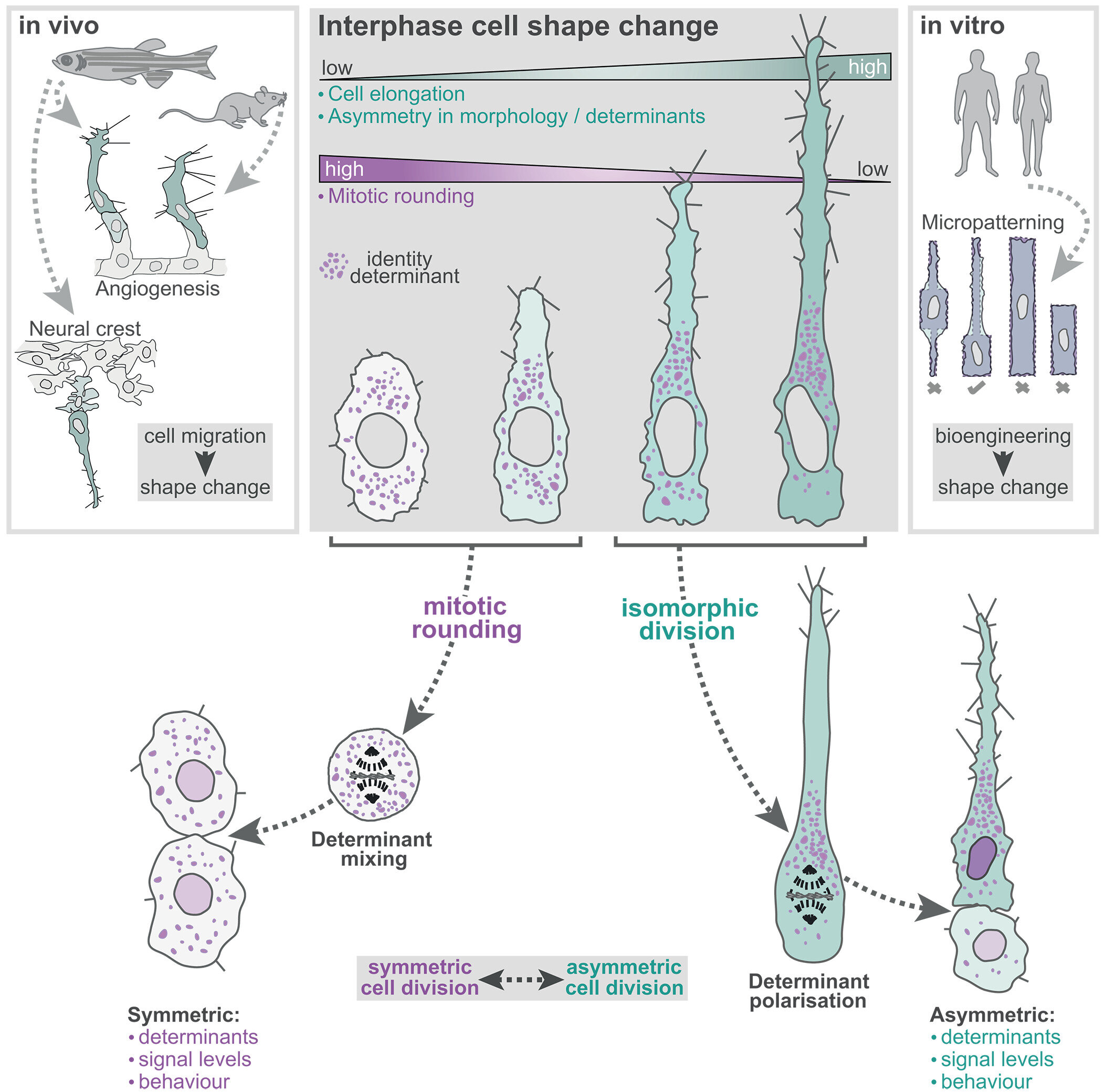
At the heart of the study lies a simple, yet profound observation: not all dividing cells become spherical. In fact, many don’t. This departure from the textbook version of mitosis leads to something far more biologically powerful—asymmetric division. Rather than producing two identical daughter cells, this form of division results in progeny that differ not just in size but also in their fate and function.
Asymmetric division has long been understood as a hallmark of stem cells—those elite units of biological potential that can become anything from neurons to muscle tissue. But the new research reveals that this type of division isn’t confined to stem cells alone. It’s a widespread phenomenon, occurring throughout tissues in developing organisms, and it all hinges on a surprising factor: the shape of the parent cell before division.
Shape Dictates Fate
The team discovered that the initial geometry of a cell—whether it is round and squat or elongated and narrow—sets the stage for how it will divide. Cells that are short and wide tend to follow the expected path: they round up and split into two nearly identical daughter cells. But cells that are long and thin don’t conform. These cells bypass the rounding stage and go on to divide asymmetrically, producing daughter cells that are distinct from one another.
This shape-driven mechanism appears to be nature’s way of breaking the symmetry of division, introducing diversity into tissues, and enabling the formation of complex structures in the body. One daughter cell may inherit the properties necessary to become a nerve fiber, while the other becomes part of a supporting structure. In a biological sense, form truly determines function—even before the cell divides.
Zebrafish Reveal the Truth
To visualize this phenomenon in real time, the researchers turned to a tiny but powerful model: the transparent embryo of the zebrafish. Just one day old, these embryos are nearly invisible and allow scientists to peer directly into the machinery of life as it unfolds.
In their study, the scientists focused on angiogenesis—the growth of new blood vessels from pre-existing ones. These growing vessels resemble living rivers, formed by lines of migrating cells. At the forefront of each growing vessel is a “tip cell”—fast, active, and exploratory. Trailing behind are the slower “stalk cells.”
Here’s where it gets interesting: when a tip cell divided, it didn’t behave as the textbooks suggested. It didn’t round up. Instead, it retained its elongated form and divided asymmetrically. One daughter cell retained the “tip” identity, racing ahead to lead the vessel, while the other became a slower follower. The researchers had visual confirmation: asymmetric division was not only real, but essential for tissue growth and patterning.
Micropatterning the Future of Cell Biology
To dive deeper into the shape-division connection, the team turned to human cells and employed a state-of-the-art technique known as micropatterning. This method involves printing protein shapes onto a surface using a UV laser, then seeding cells onto these microscopic templates. Cells grow only in the areas defined by the laser, adopting the exact shape designed by the researchers.
Using the PRIMO system by Alvéole, the team shaped cells into long rectangles, tight circles, or other precise geometries—smaller than a tenth the width of a human hair. They could then observe how each shape influenced the mode of cell division.
What they saw was crystal clear: geometry alone was enough to control whether a cell would divide symmetrically or asymmetrically. A circular template led to the expected, equal daughter cells. But elongated shapes produced divisions that were not just unequal in size, but dramatically different in behavior.
Implications for Cancer and Regeneration
This breakthrough doesn’t just rewrite the biological narrative—it could also transform how we approach disease and healing.
In the context of cancer, for instance, cells that divide asymmetrically might generate subpopulations with different behaviors. Some may remain within the tumor, while others take on properties that promote metastasis, spreading cancer to other parts of the body. Understanding and manipulating cell shape could one day provide a new avenue for halting cancer in its tracks.
On the flip side, the implications for regenerative medicine are equally exciting. If scientists can guide cell fate simply by altering cell shape, they may one day be able to custom-build tissues for transplants or organ repair. Want to generate a neuron instead of a glial cell? Start with a long, narrow progenitor. Need uniform muscle cells? Begin with round templates.
Rethinking Biology Education
Dr. Shane Herbert, senior research fellow and co-lead author, emphasized how these findings may even influence biology curricula around the world. “The phenomenon of mitosis—or cell division—is one of the fundamentals of life and a basic biological concept which is taught from school age,” he noted. “Our study, however, shows that in real living organisms, it is not as simple as that.”
This new understanding underscores the need to shift away from oversimplified diagrams and into a richer, more dynamic portrayal of cellular behavior. The future of biology education will need to include the nuances of asymmetric division and the key role of geometry in cellular identity.
Visualizing the Living Process
Dr. Holly Lovegrove, a lecturer at The University of Manchester and co-lead author, emphasized the importance of watching cells divide in living systems. “Using transparent 1-day-old zebrafish embryos allows us to study a dynamic process like cell division inside a living organism,” she explained. “We are therefore able to make movies of this fundamental cell behavior, and in doing so, reveal exciting new aspects of how tissues grow.”
Indeed, their work wasn’t just theoretical—it was cinematic. Time-lapse imaging allowed them to capture every twitch, stretch, and divide, illuminating a biological process with unprecedented clarity.
The Shape of Things to Come
The results of this study suggest that the traditional idea of the “spherical cell dividing into two equal daughters” might be more exception than rule. Real life, it seems, is messier and more creative. Cells don’t always play by the rules written a hundred years ago. They are responsive, adaptive, and exquisitely sensitive to shape and context.
As the implications of this work continue to unfold, researchers are already considering how this knowledge could be harnessed. Could doctors prevent cancer metastasis by manipulating cell geometry? Could tissue engineers grow better organs by shaping cellular environments? Could educators better prepare students by teaching cell biology as a dynamic and nuanced field, rather than a static set of diagrams?
One thing is clear: this discovery doesn’t just shift our understanding of how cells divide—it reframes our appreciation for the elegance and complexity of life itself.
Reference: Holly E. Lovegrove et al, Interphase cell morphology defines the mode, symmetry, and outcome of mitosis, Science (2025). DOI: 10.1126/science.adu9628. www.science.org/doi/10.1126/science.adu9628