Across the globe, waistlines are expanding at an alarming rate. More than two billion adults are overweight, and over 650 million are classified as obese. This epidemic is not just a matter of aesthetics—obesity is one of the most dangerous risk factors for a host of chronic diseases, including type 2 diabetes, cardiovascular disease, non-alcoholic fatty liver disease, and even certain cancers.
Modern medicine has spent decades trying to develop a silver bullet for this complex condition. And yet, many anti-obesity drugs come with a catch—modest efficacy, adverse side effects, or prohibitive cost. But what if the key to safe, sustainable weight loss has been hiding for thousands of years, in the root of a medicinal plant used by ancient Chinese physicians?
A groundbreaking study led by researchers at the University of Science and Technology of China (USTC), in collaboration with scientists from the Shenzhen Institutes of Advanced Technology, has breathed new life into a centuries-old herbal remedy. Their research, published in Science Advances, suggests that halofuginone (HF)—a natural compound originally used to combat malarial fevers—could be a potent new contender in the fight against obesity.
The Ancient Roots of a Modern Breakthrough
For over 2,000 years, traditional Chinese medicine has relied on the root of Dichroa febrifuga Lour, commonly known as Changshan, to treat malaria-induced fever. It’s a bitter plant, and for good reason: nestled within its fibrous tissues is a powerful alkaloid called halofuginone.
In the past, HF’s medicinal potential was largely confined to its antiparasitic and antifibrotic properties. But in a surprising turn of events, scientists exploring the compound’s broader biological effects noticed something unexpected. In mice, HF didn’t just modulate inflammation—it caused significant weight loss, and it did so safely.
What followed was a meticulous investigation into the underlying mechanism, culminating in a discovery that could have profound implications for obesity treatment.
Unlocking the Twin Hormones of Metabolic Control
To understand how HF works, you need to step into the world of metabolic signaling—a delicate dance of hormones, proteins, and receptors that governs hunger, satiety, and how our bodies store or burn energy. At the center of this metabolic orchestra are two powerful players: GDF15 and FGF21.
Growth Differentiation Factor 15 (GDF15) is sometimes nicknamed the “starvation hormone” or “anorexigenic factor.” It acts as a satiety signal, telling the brain—specifically, a receptor in the hindbrain known as GFRAL—that the body has had enough to eat. When GDF15 levels rise, appetite decreases.
Fibroblast Growth Factor 21 (FGF21), on the other hand, is a metabolic master switch. Produced primarily in the liver, it promotes fat oxidation, enhances insulin sensitivity, and triggers thermogenesis—the process by which the body burns calories as heat.
Remarkably, HF was shown to boost the endogenous production of both GDF15 and FGF21, essentially striking at the two main pillars of energy balance: intake and expenditure.
A Dual-Action Approach to Weight Loss
This dual-hormone effect is what makes HF so intriguing. Most weight-loss drugs target either the appetite center (like GLP-1 agonists) or energy expenditure (such as thermogenic agents), but HF targets both simultaneously.
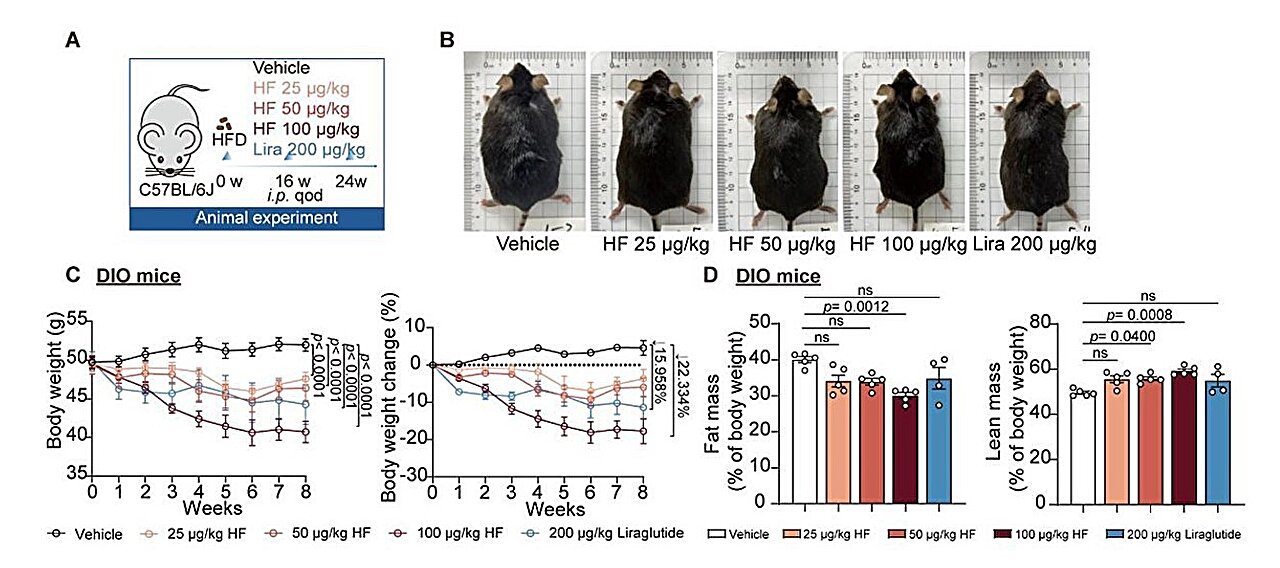
In diet-induced obese (DIO) mice—laboratory models designed to mimic human metabolic syndrome—HF administration led to dramatic reductions in body weight. Appetite was reduced thanks to increased GDF15, while FGF21 ramped up the body’s ability to burn fat. The metabolic health of the animals improved across the board: better glucose tolerance, lower insulin resistance, and reduced fat accumulation in the liver.
And the benefits weren’t limited by sex, species, or environmental conditions. Whether the subjects were male or female, mice or minipigs, warm or cold housed—the effects were consistent and robust.
In the context of obesity pharmacotherapy, this kind of universal efficacy is rare.
From Gene to Protein: Targeting EPRS1
But how exactly does HF flip the metabolic switch?
The answer appears to lie in EPRS1 (glutamyl-prolyl-tRNA synthetase 1), an enzyme involved in protein synthesis. HF acts as an EPRS1 inhibitor, triggering a cellular stress response that leads to the increased secretion of GDF15 and FGF21.
When researchers knocked down GDF15 or FGF21 production in the experimental models, the weight-loss effects of HF were only partially blocked—suggesting both are essential components of its mechanism. Conversely, similar compounds that did not inhibit EPRS1 failed to induce either hormone or any meaningful weight loss.
This finding is critical because it pinpoints EPRS1 as a novel therapeutic target—a potential entry point for new, more selective obesity treatments.
Better Than Biologics? The Promise of Small Molecules
To date, some of the most promising obesity treatments involve recombinant protein drugs—lab-manufactured versions of hormones like GDF15 or FGF21. These biologics can be effective, but they’re expensive to produce, require injections, and often have short half-lives in the body.
By contrast, small molecule drugs like HF offer distinct advantages: oral bioavailability, lower production costs, and easier administration. If HF or its derivatives can safely and reliably raise endogenous levels of these hormones, they might offer a practical, scalable solution to weight management.
This is not a hypothetical scenario. The pharmaceutical world is already racing to find small molecules that act on these exact pathways. HF just might be the head start everyone’s been waiting for.
Safety and Efficacy: A New Standard?
Safety remains a major hurdle for anti-obesity drugs. Past medications, such as fen-phen or rimonabant, were pulled from the market due to dangerous side effects ranging from heart valve damage to severe psychiatric issues. So far, HF appears to sidestep these pitfalls.
The preclinical studies reported no significant toxicity, even at effective doses. The weight-loss effects were consistent without signs of cachexia, a wasting syndrome that can accompany extreme appetite suppression. Importantly, metabolic improvements occurred without compromising lean muscle mass, a key challenge in weight loss therapy.
Still, it’s early. Human trials will be essential to confirm these findings and rule out long-term complications. But the preclinical data is promising, perhaps even unprecedented.
A Triumph of Cross-Disciplinary Discovery
One of the most fascinating aspects of this research is how it bridges ancient medicine and modern molecular biology. A compound isolated from a 2,000-year-old antimalarial herb may soon be the foundation of a next-generation anti-obesity drug.
This underscores the importance of ethnopharmacology—the study of traditional medicines using modern scientific techniques. It’s a field often underfunded and undervalued, yet it holds an immense, largely untapped reservoir of therapeutic possibilities. The journey of HF—from an herbal antimalarial to a metabolic regulator—is a striking example of how traditional knowledge can spark scientific innovation.
Looking Forward: Challenges and Opportunities
Despite the excitement, many questions remain. Will HF prove effective in humans? Can its structure be optimized for better potency or reduced off-target effects? How will it compare to new GLP-1 receptor agonists, like semaglutide or tirzepatide, which are currently revolutionizing obesity treatment?
Moreover, the complex interplay between GDF15, FGF21, and systemic metabolism still isn’t fully understood. These hormones affect multiple organs and signaling pathways, and chronic elevation might carry unforeseen risks. Precision dosing, patient selection, and long-term monitoring will all be critical as researchers move toward clinical trials.
Still, the potential here is immense. In an era where nearly half the global population is either overweight or obese, a safe, effective, and affordable weight-loss pill could be a public health game-changer.
Conclusion: Halofuginone and the Hope for a Healthier Future
The story of halofuginone is a vivid reminder that in the quest to solve modern health problems, the past still holds powerful answers. From the highlands of China to the cutting-edge labs of CAS, HF’s journey is not just a scientific discovery—it’s a narrative of curiosity, collaboration, and the enduring relevance of ancient medicine.
In a world searching for sustainable solutions to the obesity crisis, halofuginone offers a compelling new path forward—one that targets the brain, the liver, and the very biology of hunger and heat. Whether HF becomes the next household name in medicine or simply paves the way for even better therapies, its discovery marks a crucial step toward a leaner, healthier, and more scientifically enlightened future.
Reference: Suowen Xu et al, The clinical antiprotozoal drug halofuginone promotes weight loss by elevating GDF15 and FGF21, Science Advances (2025). DOI: 10.1126/sciadv.adt3142