The Zika virus, transmitted primarily by mosquitoes, gained international attention due to its association with microcephaly, a severe birth defect marked by underdevelopment of the brain, resulting in an abnormally small head. This condition became a major concern during the Zika virus outbreak, particularly in regions where the virus was widespread. A new study, published on January 13 in mBio, offers a deeper understanding of the mechanisms behind Zika’s ability to cause such devastating effects during pregnancy. The study reveals that the Zika virus exploits a host protein called ANKLE2, which plays a critical role in brain development, to facilitate its replication. This discovery not only enhances our understanding of how Zika causes harm but also sheds light on similar viruses, such as dengue and yellow fever, that use the same protein for their replication processes.
At the heart of this study is the realization that Zika virus, unlike most viruses in its category, can cross the placenta, infecting the developing fetus and potentially causing irreversible neurological damage. As Priya Shah, an associate professor at the University of California, Davis, and senior author of the paper, noted, the Zika virus is “in the wrong place at the wrong time” when it crosses into the fetus. While the virus itself does not have the complex biological machinery to reproduce on its own, it hijacks the host’s cellular machinery—specifically, a protein crucial for brain development—to replicate and spread.
This protein, ANKLE2, is found throughout the body, but its most critical role occurs in the developing brain. ANKLE2 is known to be involved in the early stages of brain development, particularly in the fetus. However, the new findings show that Zika virus exploits this protein to form replication “factories” within host cells. In a typical viral infection, the virus uses the host’s cellular machinery to produce copies of itself. However, viruses like Zika are particularly adept at evading the immune system, and part of this ability comes from the virus’s capacity to hide in specialized structures within the cell, which are formed through the interaction between the viral NS4A protein and ANKLE2.
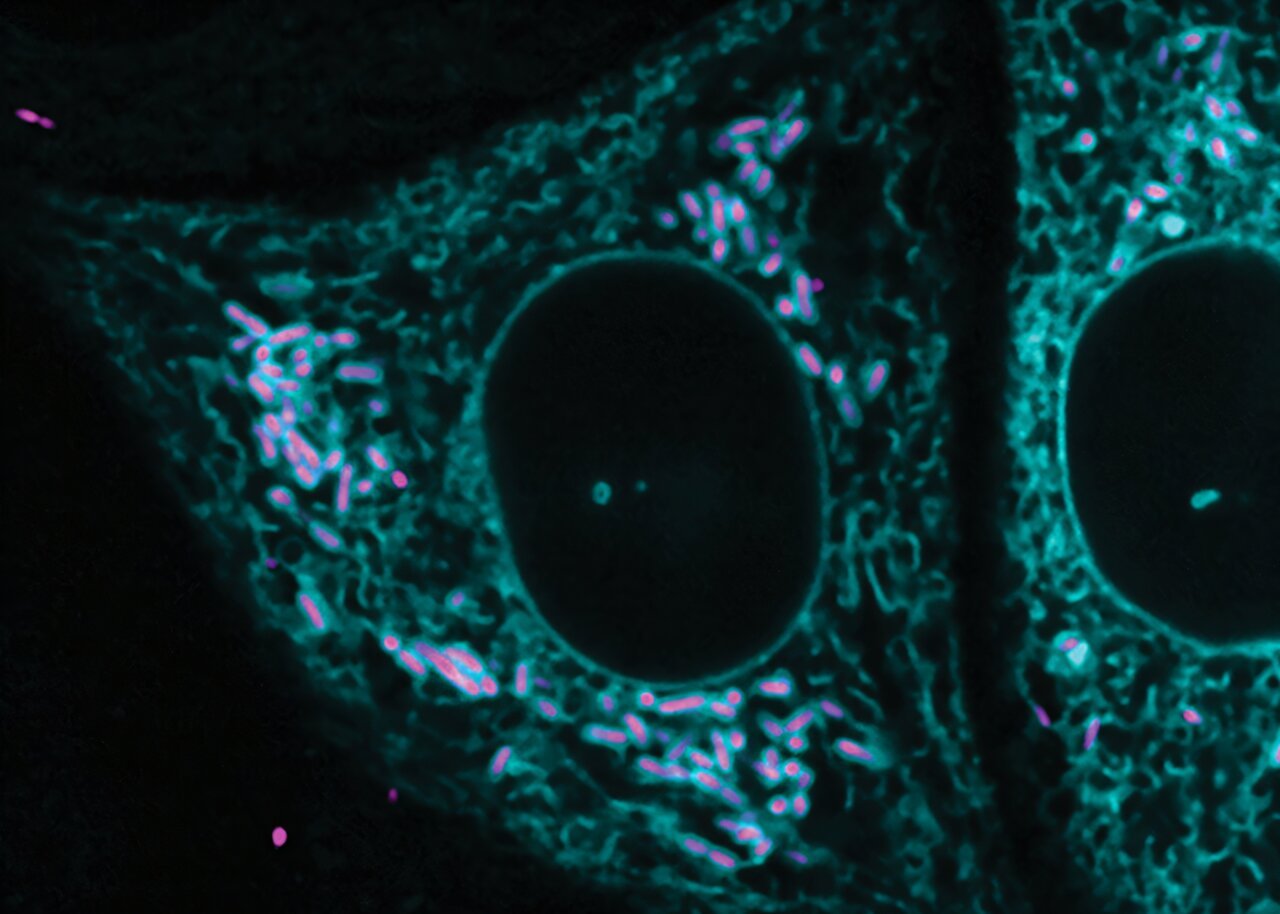
The research team, led by Ph.D. graduate Adam Fishburn, conducted experiments using human cells to investigate the relationship between Zika virus and ANKLE2. By knocking out the ANKLE2 gene in these cells, the team discovered that Zika virus replication was significantly reduced. This observation underscores the critical role of ANKLE2 in facilitating efficient viral replication. More specifically, the researchers discovered that in cells infected with Zika, ANKLE2 clusters around the endoplasmic reticulum (ER), an essential organelle responsible for protein production within the cell. The Zika virus then interacts with ANKLE2 to create small pockets or “factories” off the ER, where viral replication occurs. These pockets serve as hubs where the virus can rapidly reproduce and, importantly, shield itself from the immune system. This mechanism helps the virus evade detection by the host’s immune defenses, allowing it to replicate more effectively and spread.
ANKLE2, while not strictly essential for viral replication, is critical for the formation of these virus replication pockets. Without ANKLE2, the pockets are less efficient, which in turn reduces the virus’s ability to reproduce. As Fishburn explained, the immune system can more easily detect the virus in the absence of ANKLE2, which suggests that this protein plays a key role in the virus’s ability to evade immune surveillance.
The study also extends its focus to mosquito cells, where it was found that Zika virus also relies on ANKLE2 to replicate. This finding implies that the virus uses ANKLE2 in both human and mosquito hosts. Zika virus, as well as other related viruses like dengue and yellow fever, utilize this protein for replication, suggesting that the NS4A/ANKLE2 interaction is crucial for a broad range of mosquito-borne viruses.
What sets Zika virus apart from other related viruses, such as dengue, is its ability to cross the placental barrier, allowing it to reach the developing fetus and potentially disrupt brain development. While other viruses in the same family, like dengue and yellow fever, may also hijack ANKLE2 for replication, they do not typically cause microcephaly. This discrepancy can likely be attributed to the unique ability of Zika virus to invade the fetus. Most other viruses are unable to cross the placenta, which serves as a protective barrier to the developing baby. Zika virus’s ability to circumvent this barrier and reach the fetal brain leads to the devastating outcomes seen in infected pregnancies.
These findings open up new possibilities for therapeutic and preventive strategies against Zika and related viruses. Since the NS4A/ANKLE2 interaction is important for replication across multiple mosquito-borne viruses, targeting this interaction could serve as a potential strategy for developing antiviral treatments or vaccines. Interfering with the ability of the virus to hijack ANKLE2 could disrupt its replication process and prevent the virus from evading immune detection, ultimately reducing its ability to cause harm.
Reference: Microcephaly protein ANKLE2 promotes Zika virus replication, mBio (2025). DOI: 10.1128/mbio.02683-24