Deep within every cell of your body lies a microscopic instruction manual—your DNA. This spiral-bound library holds the blueprint for building and maintaining life. But not every instruction is followed at once. In fact, it would be catastrophic if every gene were active all the time. What determines which genes are turned on, and when? This is the intricate dance of gene expression—a marvel of biology that transforms genetic potential into biological reality.
Gene expression is more than just biology’s technical process. It’s the story of how a silent code becomes an active participant in life’s unfolding drama. It is the reason your skin cells are different from your brain cells, despite having the exact same DNA. It’s the mechanism that ensures a caterpillar becomes a butterfly, a stem cell becomes a heart, and a virus can hijack a host. It is how your cells talk, grow, adapt, and sometimes, tragically, fall apart.
To understand gene expression is to peer into the very heart of life itself. It is a story written in molecular whispers and regulated by a choreography of astonishing complexity—and elegance.
From DNA to Protein: The Central Dogma of Life
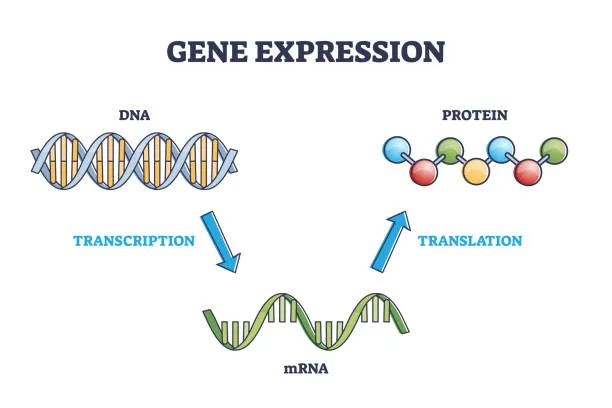
To grasp the wonder of gene expression, we begin with the central dogma of molecular biology: DNA makes RNA, and RNA makes protein. This foundational principle describes how genetic information flows within a cell.
DNA, which resides in the nucleus of eukaryotic cells, carries instructions in sequences of four chemical bases—adenine (A), thymine (T), cytosine (C), and guanine (G). These sequences form genes, each of which encodes a specific protein or functional RNA molecule.
Gene expression begins when a gene is transcribed—copied—into messenger RNA (mRNA). This mRNA strand then exits the nucleus and enters the cytoplasm, where it is translated into a chain of amino acids by ribosomes. These amino acid chains fold into proteins, the molecular machines responsible for everything from muscle contraction to immune defense to neural signaling.
But not all genes are transcribed, and not all the time. The expression of genes is meticulously regulated. It must be. The orchestration of this regulation determines the form and function of every organism.
The Layers of Regulation: A Masterpiece of Precision
Gene expression is governed by a layered system of regulation, stretching from the nucleus to the outer edges of the cell. Like a conductor managing an orchestra, the cell uses a variety of mechanisms to ensure that each gene plays at the right moment and volume.
At the transcriptional level, the first layer of control lies in deciding whether a gene’s DNA will even be transcribed into RNA. Transcription factors, proteins that bind to specific DNA sequences, act like switches. Some activate transcription by recruiting RNA polymerase—the enzyme that reads DNA and builds RNA—while others repress it.
Surrounding the DNA is chromatin, a complex of DNA and protein. Whether chromatin is open or tightly packed determines a gene’s accessibility. Epigenetic modifications—like the addition of methyl groups to DNA or acetyl groups to histones—can loosen or tighten this packaging. These changes don’t alter the DNA sequence, but they profoundly influence which genes are expressed. Remarkably, many of these epigenetic marks can be passed to daughter cells, meaning your cells can “remember” which genes to keep active.
Once an mRNA is transcribed, its fate is still uncertain. It must be processed, transported, and translated with care. The cell can modify the mRNA through alternative splicing, a process that allows a single gene to produce multiple proteins by rearranging its segments. It can also degrade mRNA molecules prematurely or prevent them from being translated—additional regulatory checkpoints that fine-tune gene output.
The final layer lies in post-translational modifications—chemical changes made to the resulting protein. These can activate, deactivate, or target the protein for destruction, further refining the effects of gene expression.
Developmental Gene Expression: Sculpting the Living Body
One of the most awe-inspiring feats of biology is the transformation of a single fertilized egg into a complex, multicellular organism. This metamorphosis is orchestrated entirely through the regulation of gene expression.
In early development, certain genes act as master regulators. These “developmental switches” control large networks of downstream genes. Homeobox (Hox) genes, for instance, dictate the body plan of animals—deciding where the head, limbs, and tail should form. These genes are incredibly conserved across species, revealing deep evolutionary roots.
As the embryo grows, signals from neighboring cells and the environment influence gene expression patterns. This interplay creates gradients of gene activity, leading to the formation of tissues and organs. Even within a single organ, different genes are activated in different cell types. This phenomenon, known as differential gene expression, is what allows the liver to produce enzymes, while neurons transmit impulses, and muscle fibers contract—all using the same genetic blueprint.
In adult organisms, gene expression continues to guide the renewal and repair of tissues. Stem cells rely on tightly controlled gene networks to maintain their identity or differentiate into specialized cells. In essence, gene expression is the thread that weaves development into the fabric of life.
Environmental Signals and Gene Expression: Adapting to the World
Gene expression isn’t set in stone. It’s remarkably responsive to the world outside. From the food you eat to the stress you endure, environmental factors continuously shape which genes are turned on or off in your cells.
Nutrient availability, for example, influences the expression of metabolic genes. The presence of glucose in the bloodstream activates insulin signaling, which in turn triggers the expression of genes that promote glucose uptake and storage.
Stress hormones like cortisol can alter gene expression in the brain, immune system, and other tissues—sometimes adaptively, sometimes harmfully. Chronic stress has been linked to altered expression of genes involved in inflammation, potentially contributing to heart disease, depression, and autoimmune conditions.
Even the microbes living in your gut can influence your gene expression. These symbiotic organisms produce metabolites that travel through your bloodstream, affecting gene activity in distant organs. This is part of the emerging field of microbiome-epigenome interactions, which may hold keys to understanding diseases ranging from obesity to Alzheimer’s.
Light, temperature, toxins, and physical activity all exert their influence as well. In plants, photoreceptor proteins sense light and activate genes for photosynthesis or flowering. In animals, exercise can boost the expression of genes involved in energy metabolism and mitochondrial biogenesis, enhancing endurance and health.
Gene expression is not a static script—it’s a living dialogue between the genome and the environment.
Epigenetics: Memory Without Mutation
In the 20th century, genetics focused largely on DNA sequence—the order of A, T, C, and G. But in recent decades, scientists have discovered that there’s another layer of information written not in the sequence, but in the packaging. This is the realm of epigenetics.
Epigenetics refers to changes in gene activity that do not involve alterations to the DNA sequence itself. These changes are often chemical tags added to DNA or histone proteins. One of the most common is DNA methylation—the attachment of a methyl group to cytosine bases in DNA. Methylation usually silences gene expression, and it’s essential in development, genomic imprinting, and X-chromosome inactivation.
Another major player is histone modification. DNA wraps around histones to form nucleosomes, and modifying these histones with chemical groups can loosen or tighten DNA’s grip, affecting accessibility.
What’s truly astonishing is that some epigenetic marks can be passed on during cell division, giving cells a memory of past experiences. This memory allows liver cells to stay liver cells, skin cells to renew themselves, and immune cells to remember pathogens.
Moreover, environmental exposures can lead to epigenetic changes that persist. Studies in mice have shown that a mother’s diet can alter the methylation patterns in her offspring, affecting coat color, weight, and disease risk. In humans, research into the Dutch Hunger Winter has found that children conceived during famine had altered methylation of genes decades later.
This opens tantalizing, and sometimes troubling, possibilities: that your lifestyle, stress levels, and nutrition could affect not just your health, but that of your descendants.
When Regulation Goes Wrong: Diseases of Gene Expression
Gene expression must be exquisitely regulated. When the system breaks down, the consequences can be devastating.
Cancer is perhaps the most dramatic example. Tumor cells often exhibit widespread dysregulation of gene expression. Genes that promote cell division (oncogenes) may become hyperactive, while those that suppress tumors (tumor suppressor genes) may be silenced—sometimes due to mutations, but often due to epigenetic changes. Abnormal methylation patterns are now recognized as hallmarks of many cancers, including colon, breast, and prostate cancers.
Neurodegenerative diseases also involve gene expression gone awry. In Alzheimer’s disease, the expression of genes related to inflammation, synaptic function, and cell death is altered. In Huntington’s disease and fragile X syndrome, expansions of DNA repeats cause gene silencing or misexpression through epigenetic mechanisms.
Autoimmune disorders like lupus and rheumatoid arthritis may result in part from misregulated gene expression in immune cells, leading them to attack the body’s own tissues.
Even common diseases like diabetes and heart disease involve the interplay between genes and their expression patterns—an interaction influenced by diet, lifestyle, and environment.
Understanding these patterns opens the door to targeted therapies. Drugs that inhibit histone deacetylases or DNA methyltransferases are being used to treat certain cancers. Personalized medicine, guided by the gene expression profile of a patient’s tumor or tissue, is becoming a reality.
RNA’s Expanding Role: Beyond Messenger
For decades, RNA was thought to be merely a messenger—a simple courier carrying DNA’s instructions to the ribosome. But RNA is now recognized as a key regulator in its own right.
Non-coding RNAs—RNAs that don’t code for proteins—play critical roles in gene expression. MicroRNAs (miRNAs), for instance, can bind to mRNA molecules and block their translation or trigger their degradation. One miRNA can regulate hundreds of genes, forming a vast, interconnected web of control.
Long non-coding RNAs (lncRNAs) are even more mysterious. Some act as scaffolds, bringing together proteins involved in gene silencing. Others seem to activate genes by altering chromatin structure. Their roles are still being discovered, but they appear vital in development, stem cell maintenance, and disease.
The realization that much of our genome is transcribed into non-coding RNAs has revolutionized our understanding of gene regulation. What was once dismissed as “junk DNA” now appears to be teeming with regulatory potential.
Future Frontiers: Engineering Expression
As our understanding deepens, scientists are beginning not just to observe gene expression—but to control it.
Synthetic biology allows researchers to design genetic circuits—combinations of genes and regulatory elements that behave like electronic circuits. These can be inserted into cells to produce specific outputs in response to stimuli. Imagine a cell that detects a tumor environment and responds by releasing a drug. Or a microbe engineered to clean up oil spills by expressing enzymes only in polluted water.
CRISPR technology, originally developed to edit DNA, is now being adapted to control gene expression. CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) use deactivated Cas9 proteins fused to regulatory domains to silence or activate genes at will. This precision holds promise for treating genetic disorders, studying development, and even enhancing agriculture.
Gene therapy, too, is evolving from brute-force replacement of faulty genes to subtle tuning of expression levels. The future may bring treatments that dial genes up or down like a volume knob—customized for each patient.
The Poetry of Expression
At its core, gene expression is a story of identity, change, and responsiveness. It is the way life turns potential into reality, silence into function, code into consciousness. It is a system so finely tuned that a cell can respond to a whisper of a signal—or resist a scream.
It is how a fertilized egg becomes a child, how a brain remembers, how an immune system learns. It is how you adapt to heartbreak, hunger, sunlight, and sleep.
And though it unfolds in the language of molecules, gene expression is nothing short of poetry—written in the stanzas of A, T, C, and G, and recited endlessly in the quiet pulse of living cells.