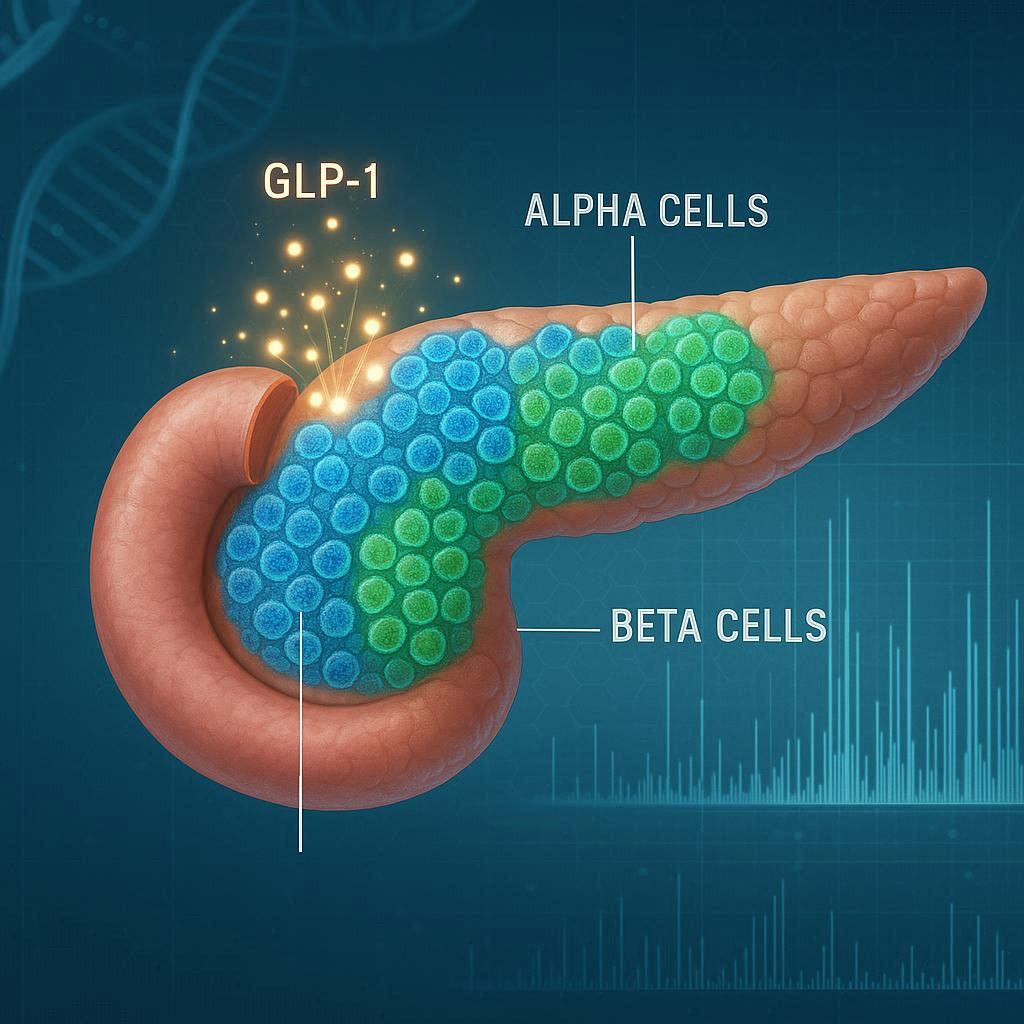
For decades, biology textbooks told a simple story: pancreatic beta cells make insulin to lower blood sugar, while alpha cells make glucagon to raise it. The two hormones balance each other, ensuring that our bodies always have the right amount of fuel for activity or rest. But new research from Duke University School of Medicine is rewriting that story—and revealing a surprising twist that could reshape how we treat type 2 diabetes.
Published in Science Advances, the study shows that alpha cells are far more versatile than anyone realized. Instead of being limited to producing glucagon, they also generate GLP-1, a hormone celebrated for its ability to boost insulin release and lower blood sugar. GLP-1 is already the target of powerful diabetes drugs like Ozempic and Mounjaro. The revelation that the pancreas itself may be a natural source of this hormone adds an entirely new dimension to our understanding of metabolism.
The Surprising Power of GLP-1
GLP-1, or glucagon-like peptide-1, is best known as a hormone produced in the gut after eating. It tells the pancreas to release insulin, slows digestion, and helps control appetite—effects that make it a powerful tool for managing diabetes and even obesity. But the Duke team, led by Jonathan Campbell, Ph.D., found that pancreatic alpha cells themselves can produce significant amounts of bioactive GLP-1.
Using cutting-edge mass spectrometry, the researchers were able to measure the hormone with unprecedented precision. What they discovered surprised even seasoned endocrinologists: human alpha cells produce far more GLP-1 than previously believed, and this production directly stimulates insulin release.
“Alpha cells are more flexible than we imagined,” Campbell explained. “They can adjust their hormone output to support beta cells and maintain blood sugar balance.”
This discovery suggests that the pancreas has its own built-in backup system—one that may help compensate when beta cells struggle, as they do in type 2 diabetes.
A Switch Between Hormones
The study didn’t just reveal that alpha cells can make GLP-1; it showed that these cells can actually switch their hormone production depending on the body’s needs. In mouse studies, when researchers blocked glucagon production, they expected the loss of signaling between alpha and beta cells to weaken insulin release. Instead, the opposite happened.
Deprived of glucagon, alpha cells increased GLP-1 production. The result was stronger insulin release, better glucose control, and improved communication between cells.
“It was the opposite of what we thought,” Campbell said. “When glucagon was suppressed, GLP-1 took over—and it turned out to be an even better stimulator of insulin than glucagon.”
This flexibility, or “switching,” demonstrates a hidden resilience in the pancreas. Far from being rigid, alpha cells can pivot their role, offering the body a second line of defense against rising blood sugar.
Decoding the Enzyme Puzzle
The team also investigated how alpha cells decide whether to produce glucagon or GLP-1. Two key enzymes control this choice: PC2 drives glucagon production, while PC1 leads to GLP-1.
By blocking PC2, researchers were able to tip the balance toward PC1, resulting in more GLP-1 and better blood sugar control. But when both enzymes were shut down, insulin secretion faltered, and blood sugar spiked. This confirmed just how critical GLP-1 is for maintaining healthy glucose levels.
The discovery hints at potential therapeutic strategies: if scientists can safely manipulate these enzyme pathways, they may be able to naturally boost GLP-1 production inside the pancreas itself.
Implications for Diabetes Treatment
Type 2 diabetes arises when beta cells can no longer produce enough insulin to keep up with rising blood sugar. Current treatments include medications that stimulate insulin, increase sensitivity to it, or mimic GLP-1. But the idea that the pancreas can be coaxed into making more GLP-1 on its own could open the door to new therapies that work with the body’s natural systems rather than overriding them.
While GLP-1 medications are effective, they can be expensive and sometimes cause side effects. A therapy that enhances the pancreas’s own GLP-1 production could provide a more sustainable, physiologically aligned approach.
Interestingly, the researchers also found that common stressors, such as a high-fat diet, modestly increased alpha cell GLP-1 production. While this alone isn’t enough to prevent diabetes, it suggests that the body already tries to adapt in times of metabolic stress—though often not powerfully enough to prevent disease progression.
The Challenge of Measurement
One reason this discovery has remained hidden until now is that GLP-1 is notoriously difficult to measure. The hormone exists in multiple forms, many of them inactive fragments that don’t actually affect insulin secretion. Past studies often picked up these fragments, muddying results.
To overcome this, the Duke team developed a highly specific mass spectrometry assay capable of detecting only the bioactive form of GLP-1—the version that truly stimulates insulin. This breakthrough in measurement was key to uncovering the hormone’s unexpected presence and abundance in alpha cells.
“This shows that the body has a backup plan,” Campbell noted. “GLP-1 is simply a much more powerful signal for beta cells than glucagon. The ability to switch from glucagon to GLP-1 in times of stress may be essential for maintaining blood sugar control.”
A New Frontier in Metabolic Research
This study is more than a scientific curiosity—it’s a paradigm shift. For years, alpha cells were seen as antagonists to beta cells, working in opposition by raising blood sugar while beta cells tried to lower it. Now, it appears that alpha cells can act as allies, stepping in to produce GLP-1 when the body needs extra insulin support.
The implications are profound. If researchers can learn how to safely amplify this natural flexibility, it could lead to therapies that restore balance without overwhelming the system. Such treatments might one day delay or even prevent the onset of type 2 diabetes in at-risk individuals.
As with all scientific discoveries, questions remain. How do alpha cells decide when to switch from glucagon to GLP-1? Can this process be harnessed safely in humans? And how might this discovery interact with existing GLP-1-based drugs?
What’s clear is that our understanding of the pancreas is still evolving. The organ once thought of as a simple on–off switch for blood sugar turns out to be a far more nuanced and adaptive system.
Conclusion: Rediscovering the Pancreas
The Duke University study reveals a remarkable truth: the pancreas is not merely a battleground between insulin and glucagon, but a dynamic, adaptive system with hidden reserves of resilience. Alpha cells are not just the “opposite” of beta cells; they are versatile partners, capable of stepping into a supportive role by producing GLP-1.
This discovery challenges decades of conventional wisdom and offers hope for new ways to tackle type 2 diabetes. It underscores the body’s own ingenuity in maintaining balance—and our growing ability to uncover its secrets.
As scientists continue to explore this hidden capacity, one thing becomes clear: the story of blood sugar regulation is far richer, more complex, and more promising than we ever imagined.
More information: Canqi Cui et al, Alpha-cells use both PC1/3 and PC2 to process proglucagon peptides and control insulin secretion, Science Advances (2025). DOI: 10.1126/sciadv.ady8048. www.science.org/doi/10.1126/sciadv.ady8048