Every day, the cells in our bodies are bombarded by harmful influences — ultraviolet rays from sunlight, environmental toxins, metabolic byproducts, and even random errors during cell division. Much of this invisible warfare plays out at the level of DNA, the molecule that carries the instructions of life. Over time, the accumulation of DNA damage is known to drive both aging and cancer. Yet the connection between these two outcomes — the slow drift into cellular decline versus the explosive rise into malignancy — has remained one of biology’s most compelling mysteries.
A new study published in Nature Cell Biology offers a rare key to that puzzle. Focused on melanocyte stem cells (McSCs) — the cells that replenish pigment-producing melanocytes in hair follicles — researchers from the University of Tokyo have shown that the same population of cells can travel down two radically different paths depending on what kind of DNA injury they sustain and what signals they receive from their environment. One path leads to harmless aging and hair graying. The other sets the stage for melanoma, one of the deadliest forms of skin cancer.
Stem Cells That Carry Color — and Risk
Melanocyte stem cells live in the quiet, protected zone of the hair follicle, serving as a reservoir for pigment cells that give color to skin and hair. Under normal circumstances, they remain immature, regenerating with each hair cycle. But unlike short-lived mature cells, stem cells are long-term residents — their responses to long-term DNA damage matter enormously for future tissue health.
Using long-term lineage tracing in live mice, combined with gene expression profiling, the research team led by Emi Nishimura and Yasuaki Mohri asked a deceptively simple question: When these pigment stem cells are damaged, what do they do?
When Cells Choose Sacrifice Over Risk
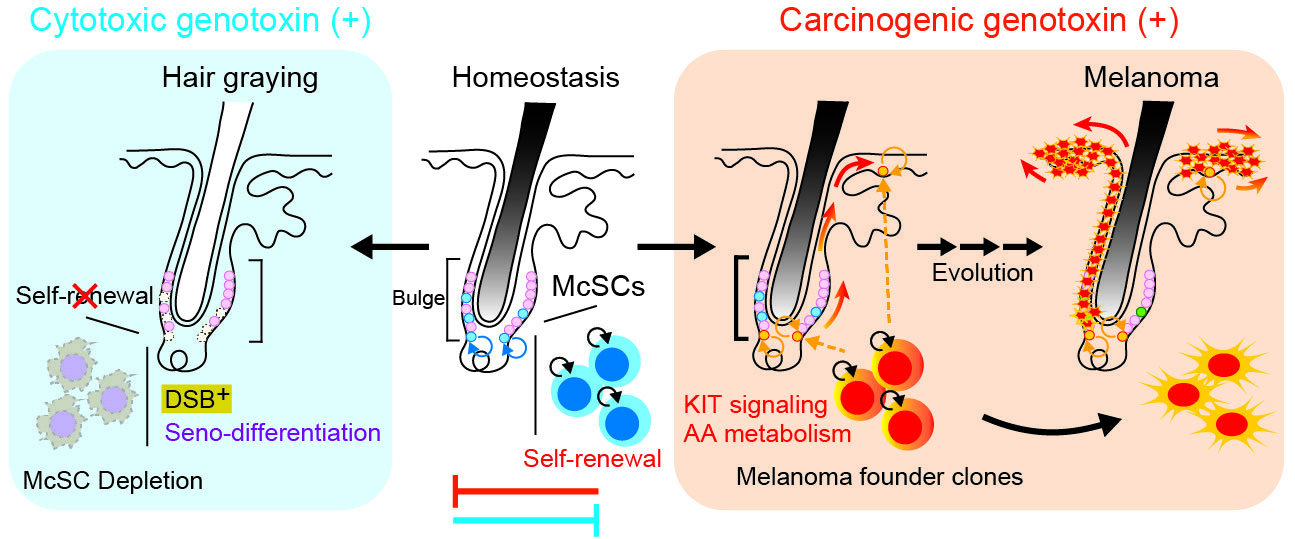
The team observed that when McSCs experience severe DNA double-strand breaks — one of the most catastrophic types of genetic injury — they activate a defensive program. Through the p53–p21 signaling pathway, the cells enter a state of senescence-linked forced differentiation, a response the authors call seno-differentiation. In essence, the stem cells “choose” to mature prematurely and exit the stem cell pool forever.
This protective self-sacrifice has a visible side effect: hair loses pigment because the stem cell reservoir is depleted, resulting in graying. But beneath the cosmetic change lies a profound biological act — tissues remove damaged, mutation-prone stem cells before they can turn cancerous.
Graying hair, in this light, is not a passive feature of aging but an active defense — a visible trace of microscopic risk management.
When the System Fails to Shut Down
Not all DNA damage invokes this protective pathway. When McSCs were exposed to carcinogens such as ultraviolet B or the chemical 7,12-dimethylbenz[a]anthracene, the cells behaved differently. Despite carrying DNA damage, they bypassed the seno-differentiation checkpoint. Instead of maturing and being removed, they continued to self-renew — and even expanded.
That persistence was not accidental. The researchers found that signals from the surrounding microenvironment — particularly KIT ligand secreted from the follicle niche and epidermis — actively suppressed the protective differentiation program. The niche effectively told damaged cells to stay alive and keep dividing, creating fertile ground for tumor initiation.
In this scenario, damaged stem cells are not eliminated but preserved — and a seed of melanoma risk is planted.
Two Outcomes, One Root Cause
These findings dissolve the apparent separation between hair graying and cancer. They reveal them as two contrasting consequences of how the same stem cells respond to stress:
When the defensive route dominates, damaged cells eliminate themselves, leading to gray hair and protection.
When microenvironmental cues suppress this route, damaged cells expand, raising the likelihood of melanomagenesis.
The study does not imply that people with gray hair are “safe” from cancer or that delaying graying increases cancer risk. Rather, it reframes graying as one instance of a larger biological principle: sometimes aging is a side effect of a built-in self-cleansing strategy.
Aging as a Form of Protection
A central conceptual contribution of this work is that aging and cancer are not opposite destinies but intertwined responses to DNA damage. Aging often emerges when tissues favor safe exhaustion — removing compromised stem cells through natural senolysis. Cancer emerges when tissues instead retain damaged stem cells and allow them to expand.
In this respect, aging may sometimes be the price of survival — the visible toll of invisible decisions that keep dangerous cells from taking over. The loss of pigment that gives rise to white hair can be seen as an outward symbol of the body’s choice of safety over risk.
A New Framework for Tissue Health
By identifying the molecular switches and niche signals that push cells toward either exhaustion or expansion, the study offers a framework for understanding how the same damage can yield life’s quiet fading or its most violent rebellion. It suggests that future therapies — for both melanoma and age-related degeneration — may one day hinge on learning how to modulate these fate decisions.
The findings echo a deeper biological truth: sometimes the body ages because it is wisely protecting itself. The difference between graying and cancer may lie not in whether damage occurs, but in how cells decide to respond — whether they choose to die with dignity or to gamble with the future.
More information: Yasuaki Mohri et al, Antagonistic stem cell fates under stress govern decisions between hair greying and melanoma, Nature Cell Biology (2025). DOI: 10.1038/s41556-025-01769-9