From the moment we wake up to the time we go to sleep, our world is governed by balance. Our bodies regulate temperature, the economy juggles supply and demand, and ecosystems strive to maintain harmony. But nowhere is balance more deeply ingrained than in the invisible realm of chemistry.
In chemistry, this balance is called equilibrium—a state where opposing processes occur at the same rate, resulting in no net change. It’s the molecular equivalent of a perfectly poised scale or a tightrope walker frozen in place. But chemical equilibrium is anything but still. Beneath its calm surface lies a dynamic tug-of-war, a silent contest of rates, molecules, and energies.
Equilibrium is more than just an academic concept tucked away in textbooks. It’s a powerful idea that shapes reactions in our bodies, industrial processes, the environment, and even the stars. Whether you’re baking bread, breathing air, or studying rocket fuel, you are intimately tied to this concept.
This article takes you on a deep, exploratory journey into the heart of chemical equilibrium. Along the way, you’ll learn not only what equilibrium is, but also how it works, why it matters, and how it defines the very chemistry of life.
Understanding Chemical Reactions: A Prelude to Equilibrium
To understand equilibrium, we must first understand chemical reactions themselves. In any reaction, substances known as reactants are transformed into products. This transformation is driven by the rearrangement of atoms, breaking and forming bonds, and a shift in energy.
For example:
H₂ + I₂ ⇌ 2HI
In this reaction, hydrogen and iodine combine to form hydrogen iodide. But notice the special symbol in the middle—⇌. This is the symbol of a reversible reaction.
Unlike a one-way street, many reactions in chemistry are reversible. This means that the products can also react to reform the reactants. In our example, hydrogen iodide can decompose back into hydrogen and iodine.
And here’s the key idea: when the forward and reverse reactions occur at the same rate, the system reaches equilibrium.
Defining Chemical Equilibrium: The Dynamic Balance
At chemical equilibrium, the concentrations of reactants and products remain constant over time—not because the reaction has stopped, but because the forward and reverse reactions occur at equal rates.
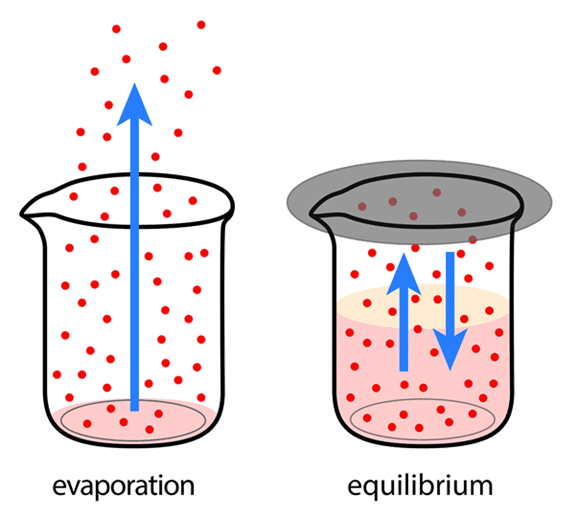
This is a subtle but profound idea. Imagine pouring water between two cups. At first, water rushes from one to the other. But eventually, the levels even out. Water still moves between the cups, but at equal rates in both directions. This is what dynamic equilibrium looks like.
Importantly, equilibrium does not mean equal amounts of reactants and products. It simply means that their concentrations are unchanging.
Depending on the reaction, the equilibrium position can favor the products (meaning mostly products exist at equilibrium), or the reactants (meaning little reaction has occurred). This “position” of equilibrium is determined by energy, molecular stability, and other factors.
The Equilibrium Constant: A Window into the Reaction
To quantify equilibrium, chemists use a powerful tool: the equilibrium constant, denoted K.
For a general reaction:
aA + bB ⇌ cC + dD
The equilibrium constant is given by:
K = [C]^c [D]^d / [A]^a [B]^b
Here, the brackets [ ] represent concentrations, and the lowercase letters are coefficients from the balanced equation. This ratio compares the concentrations of products to reactants at equilibrium.
If K > 1, products are favored.
If K < 1, reactants are favored.
If K ≈ 1, the system has significant amounts of both.
Each reaction has its own K value, dependent on temperature, and it provides profound insight into the behavior of the system. A massive K suggests near-complete conversion, while a tiny K implies hardly any reaction takes place.
Le Chatelier’s Principle: Predicting the System’s Response
Imagine you’re watching a perfectly balanced scale, and suddenly someone adds weight to one side. The balance is disturbed. What happens next? The system readjusts until balance is restored.
In chemistry, Le Chatelier’s Principle describes this beautifully.
This principle states that if a system at equilibrium is disturbed by a change in concentration, pressure, temperature, or volume, the system will shift its equilibrium position to counteract the disturbance.
Let’s unpack this with some examples:
Change in Concentration
In the reaction:
N₂ + 3H₂ ⇌ 2NH₃
If we add more nitrogen, the system shifts right to consume the added nitrogen and produce more ammonia.
If we remove hydrogen, the system shifts left to restore the lost reactant.
Change in Pressure
For gaseous reactions, changes in pressure affect equilibrium if the number of gas molecules changes.
2NO₂ ⇌ N₂O₄
If we increase pressure (by decreasing volume), the system shifts toward fewer moles of gas—in this case, to the right.
Change in Temperature
Temperature changes affect the value of K.
In exothermic reactions (releases heat), adding heat shifts the equilibrium left.
In endothermic reactions (absorbs heat), adding heat shifts the equilibrium right.
This thermodynamic dance explains why reactions behave differently at various temperatures and helps chemists manipulate yields.
The Reaction Quotient: Snapshot of Progress
Before equilibrium is reached, we can assess the system using the reaction quotient, Q.
It’s calculated the same way as K, but uses current concentrations instead of equilibrium concentrations.
- If Q < K, the reaction shifts right.
- If Q > K, the reaction shifts left.
- If Q = K, the system is at equilibrium.
This tool is vital in industry and labs to determine how far a reaction must proceed before reaching stability.
Types of Equilibrium: Physical, Chemical, and Beyond
Equilibrium isn’t limited to chemical reactions. It appears in a variety of forms:
Physical Equilibrium
Occurs in phase changes:
H₂O (l) ⇌ H₂O (g)
Here, the rate of evaporation equals the rate of condensation. The water level stays the same, but molecules continuously switch states.
Solubility Equilibrium
AgCl (s) ⇌ Ag⁺ (aq) + Cl⁻ (aq)
In saturated solutions, undissolved solids are in equilibrium with their dissolved ions. The solubility product constant (Ksp) quantifies this balance.
Acid-Base Equilibrium
CH₃COOH ⇌ CH₃COO⁻ + H⁺
Weak acids and bases establish equilibrium with their ions. The strength of an acid depends on its Ka, the acid dissociation constant.
These types reveal the broad power of equilibrium in everything from solutions to respiration.
Industrial Applications: Chemistry Meets Economics
In industrial chemistry, equilibrium is more than theory—it’s money. Consider the Haber Process, which synthesizes ammonia:
N₂ + 3H₂ ⇌ 2NH₃ + heat
This reaction has a small K and is exothermic. By manipulating temperature and pressure, industrial chemists push the reaction toward higher yields of ammonia.
They increase pressure to favor the side with fewer gas molecules, and lower temperature to favor the exothermic direction—though too low a temperature slows the reaction. The result is a compromise: moderate temperature, high pressure, and catalysts for efficiency.
This process feeds the world, producing fertilizers for agriculture.
Other examples include:
- Contact Process for sulfuric acid.
- Methanol Synthesis
- Polymerization reactions
Understanding equilibrium allows industries to maximize product while minimizing cost and waste.
Biological Relevance: Life in Balance
Your body is a temple of equilibrium. Every breath, heartbeat, and chemical signal depends on it.
Oxygen Transport
Hemoglobin binds oxygen in the lungs:
Hb + O₂ ⇌ HbO₂
In tissues with low oxygen, the equilibrium shifts left, releasing oxygen. In lungs, the high oxygen pushes equilibrium right. This reversible binding keeps us alive.
Acid-Base Balance
Blood pH is tightly regulated:
CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻
This buffer system maintains pH around 7.4. If blood becomes too acidic or alkaline, equilibrium shifts to restore balance.
Enzyme Reactions
Many biochemical reactions are reversible. Metabolic pathways like glycolysis and the Krebs cycle rely on equilibrium to guide energy flow.
Without equilibrium, life would be chaos. Too much of one molecule, too little of another—and the system collapses.
Equilibrium and Thermodynamics: The Energy Connection
Why do some reactions reach equilibrium while others don’t? The answer lies in thermodynamics.
Every reaction involves two key forces:
- Enthalpy (ΔH) – the heat content.
- Entropy (ΔS) – the disorder or randomness.
Together, they define Gibbs Free Energy (ΔG):
ΔG = ΔH – TΔS
At equilibrium, ΔG = 0.
- If ΔG < 0, the reaction is spontaneous.
- If ΔG > 0, the reaction is non-spontaneous.
Reactions naturally move toward lower free energy—a state of maximum entropy and minimum enthalpy. This is why equilibrium is often called the most stable state of a system.
Shifting Perspectives: When Equilibrium Isn’t Desirable
Interestingly, in some cases, chemists avoid equilibrium.
In combustion, photosynthesis, and energy storage, we want reactions to go one way.
For example, burning gasoline:
C₈H₁₈ + O₂ → CO₂ + H₂O
This is not reversible in practice. It releases energy and moves to completion. Such reactions are called irreversible, though technically, some reverse reaction exists—just so unfavorable it’s negligible.
Designing systems to prevent equilibrium is crucial in batteries, propulsion, and food preservation.
Misconceptions About Equilibrium
Even advanced students harbor misconceptions:
- “At equilibrium, all reactions stop.” False. It’s dynamic—reactions continue at equal rates.
- “Equal concentrations mean equilibrium.” Not necessarily. The rates matter, not the amounts.
- “Equilibrium always favors products.” Many reactions favor reactants.
Clarifying these concepts is key to mastering equilibrium.
Conclusion: Harmony in the Molecular World
Chemical equilibrium is a profound principle that touches every corner of science and life. From the smallest ions in solution to the workings of your own bloodstream, equilibrium governs how molecules behave, interact, and coexist.
It is not about stillness, but about balance in motion. It is not a single event, but a continuous process. It allows us to predict the outcomes of reactions, to harness chemistry for human progress, and to understand the subtle order that underlies apparent chaos.
So the next time you sip soda, cook dinner, take a breath, or gaze at a star, remember: you are witnessing equilibrium in action. The world doesn’t just spin—it balances.
And in that balance, we find the essence of chemistry itself.