Hydrogen is often celebrated as the fuel of the future. It is clean, abundant, and capable of powering everything from ships to aircraft, from heavy industry to home heating. Yet hydrogen carries with it a paradoxical danger: it weakens the very metals designed to contain and transport it. This phenomenon, known as hydrogen embrittlement, has long haunted engineers. It causes metals like steel to become brittle, crack, and sometimes fail without warning.
For decades, scientists have known that hydrogen interacts with metals in mysterious ways, but they could not watch it happen in real time. The atom itself is notoriously difficult to detect, slipping invisibly through microscopic gaps in crystal structures. That is why a recent study led by researchers from the University of Oxford and Brookhaven National Laboratory marks such a profound leap forward.
In a world-first experiment, the team revealed how hydrogen alters the inner workings of stainless steel, unlocking a new chapter in the science of materials. Their findings, published in Advanced Materials, do more than explain a long-standing mystery. They point toward safer hydrogen fuel systems for the future of clean energy.
Watching Atoms Dance in Real Time
At the heart of this discovery lies a question that has puzzled engineers: how exactly does hydrogen interact with the hidden flaws inside steel? These flaws, called dislocations, are tiny irregularities in the otherwise ordered arrangement of atoms. Though invisible to the naked eye, dislocations play a vital role in how metals bend, stretch, and ultimately break.
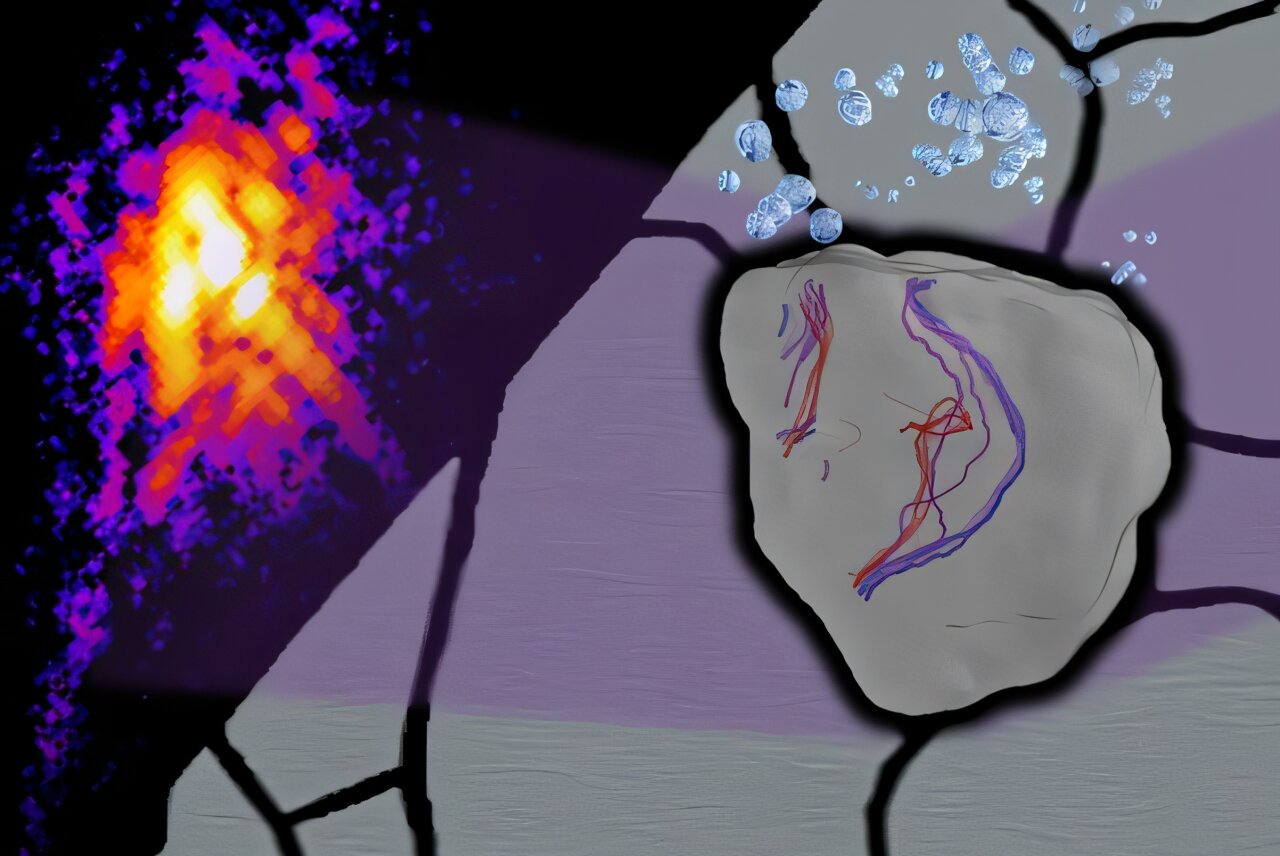
To see what hydrogen does to these dislocations, the research team used one of the brightest scientific tools on Earth: an ultra-bright X-ray beamline at the Advanced Photon Source in the United States. By focusing this powerful beam onto a stainless-steel grain less than a thousandth the width of a human hair, they were able to capture a detailed picture of the metal’s inner structure.
Using a technique known as Bragg Coherent Diffraction Imaging, the scientists tracked how the steel’s atomic lattice shifted and strained as hydrogen entered the material. For twelve hours, they recorded the subtle yet profound changes unfolding inside the crystal, producing the first-ever three-dimensional movie of hydrogen’s effects at the atomic scale.
Surprising Movements Beneath the Surface
What the researchers discovered was both unexpected and illuminating. As hydrogen atoms diffused into the steel, the dislocations—normally sluggish and constrained—suddenly became mobile. They began moving and reshaping themselves even without external stress applied. It was as though hydrogen acted like a lubricant at the atomic level, unlocking motion that should not have been possible under normal conditions.
Even more striking was the observation of a rare movement called climb. Typically, dislocations move only along certain directions within a crystal. But with hydrogen present, the defects shifted upward, out of their usual plane. This phenomenon, which usually requires high temperatures, occurred at room temperature in the experiment. It suggests that hydrogen makes it possible for atoms to rearrange themselves in ways previously thought impossible under everyday conditions.
Finally, the researchers observed a dramatic reduction in the strain fields around dislocations. These strain fields are zones where atoms are compressed or stretched to accommodate a defect. By weakening these fields, hydrogen effectively shields the surrounding lattice from stress—a phenomenon known as hydrogen elastic shielding. This effect had been theorized for years, but the Oxford-Brookhaven team delivered the first direct experimental proof.
Together, these observations explain why hydrogen can cause metals to fail. By mobilizing defects and softening the lattice around them, hydrogen undermines the steel’s strength from within, setting the stage for sudden and catastrophic fractures.
A Breakthrough for the Hydrogen Economy
These findings carry enormous implications for the future of clean energy. As nations race to build hydrogen-powered infrastructure—pipelines, storage tanks, fuel cells, and even hydrogen-fueled aircraft—the question of material reliability is paramount. A single unexpected crack in a pipeline or pressure vessel could have devastating consequences.
Lead researcher Dr. David Yang of Brookhaven National Laboratory captured the urgency of the discovery: “Hydrogen has great potential as a clean energy carrier, but it’s notorious for making materials more brittle. For the first time, we have directly observed how hydrogen changes the way defects in stainless steel behave deep inside the metal. This knowledge is essential for designing alloys that are more resilient in extreme environments.”
In other words, this study doesn’t just solve a scientific puzzle—it provides a roadmap. By understanding how hydrogen interacts with defects, engineers can design new alloys that resist embrittlement, ensuring that the infrastructure of a hydrogen economy is both safe and durable.
A Window Into the Future of Materials
For Professor Felix Hofmann of Oxford’s Department of Engineering Science, who led the project, the excitement lies not only in the results but also in the method: “Using coherent X-ray diffraction, we were able to watch atomic-scale events unfold in real time inside solid metal without cutting it open. Some of the results really surprised us by showing behavior we weren’t expecting.”
This ability to observe atomic processes non-destructively represents a leap forward in experimental physics. It allows scientists to piece together puzzles that were once hidden, creating a foundation for both theoretical models and practical engineering solutions.
The research team is already planning more advanced experiments, expanding the investigation to other types of defects and other metals. Each step will deepen our understanding of hydrogen’s dual nature: as both the clean fuel of tomorrow and a silent saboteur of the materials we rely on.
Toward Stronger, Smarter Alloys
The implications extend well beyond the hydrogen economy. In fields as varied as nuclear fusion, aerospace, and advanced manufacturing, the ability to design alloys with tailored resistance to embrittlement could reshape technology. Fusion reactors, for example, must endure bombardment by hydrogen isotopes under extreme conditions. Future hydrogen-powered aircraft will need materials that combine lightness with resilience.
By feeding their experimental data into multi-scale simulation frameworks, the researchers hope to bridge the gap between atomic-scale discoveries and large-scale engineering design. This integration could accelerate the creation of next-generation materials capable of withstanding the harshest environments.
Hydrogen’s Promise and Paradox
The study is a reminder of the paradox at the heart of hydrogen: it is both a beacon of hope for a carbon-free future and a formidable engineering challenge. Its smallest atom holds both the key to clean energy and the seeds of structural failure. Unlocking its secrets requires both advanced tools and deep collaboration across institutions, as demonstrated by the partnership between Oxford, Brookhaven, Argonne National Laboratory, and University College London.
The work is far from finished. But with every breakthrough, humanity takes a step closer to building a safe, reliable, and sustainable hydrogen economy.
The Beauty of Seeing the Invisible
Beyond its practical implications, there is something profoundly moving about this research. To see hydrogen—an element invisible and elusive—reshape the hidden landscapes inside metal is to glimpse nature’s subtle artistry. The steel beneath our feet, the pipelines carrying our energy, the aircraft soaring through the sky—all are alive with atomic dramas, unfolding unseen.
By revealing these dramas for the first time, the Oxford-Brookhaven team has not only advanced science but also deepened our appreciation of the intricate, often surprising ways the universe works. In the race toward a sustainable future, it is discoveries like this that remind us of both the fragility and resilience of the materials we depend on—and of the creativity and persistence required to understand them.
More information: Direct Imaging of Hydrogen-Driven Dislocation and Strain Field Evolution in a Stainless Steel Grain, Advanced Materials (2025). DOI: 10.1002/adma.202500221