Imagine a material that flows like liquid when pushed through a needle, yet solidifies into a structure that mimics the strength and softness of living tissue once inside the body. A material that could deliver cells exactly where they’re needed, repair damaged tissues, or serve as a living model to study how diseases evolve. This is not science fiction—it is the promise of granular hydrogels, and thanks to researchers at the University of Illinois Urbana-Champaign, that promise is coming into sharper focus.
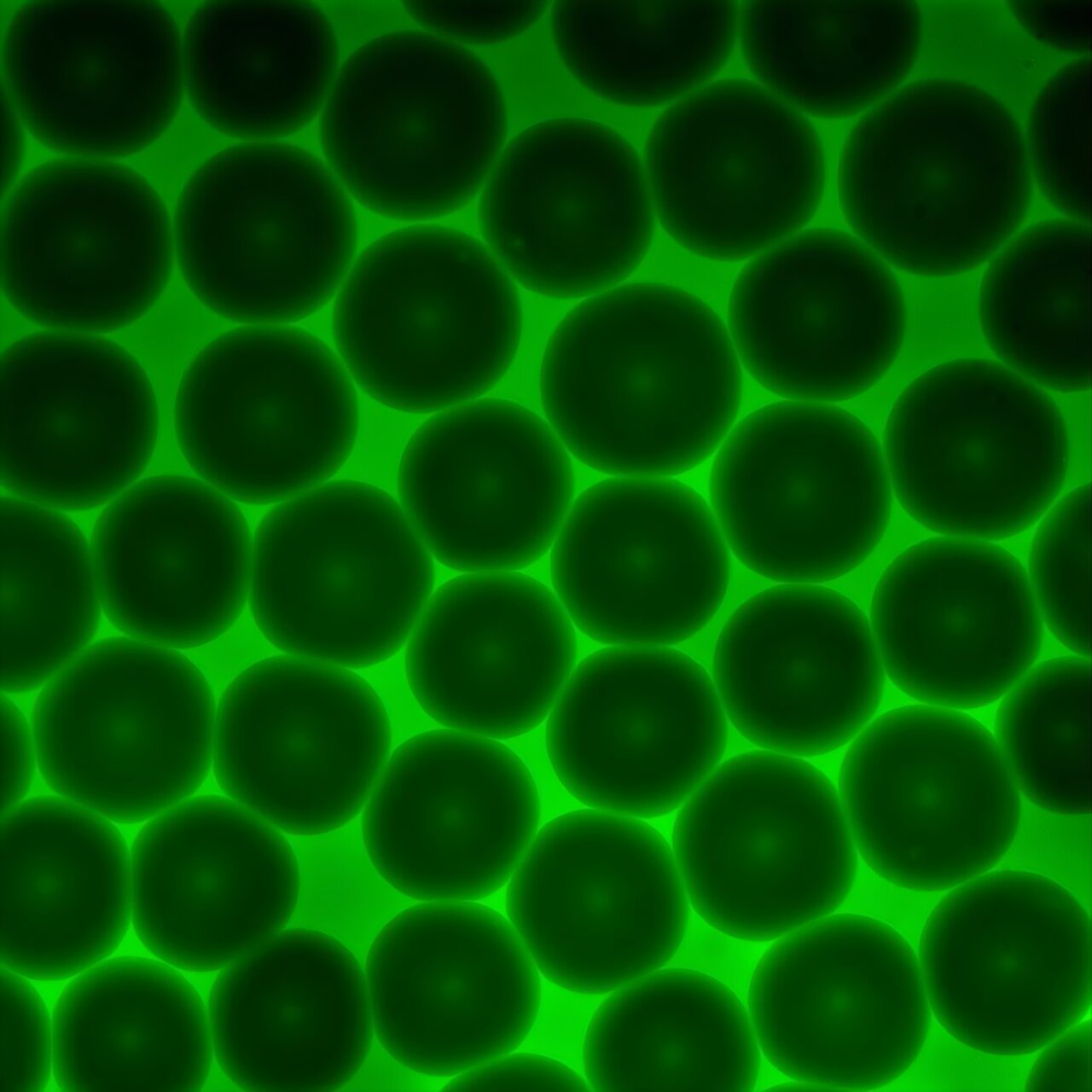
Granular hydrogels are made of densely packed microscopic gel particles. To the naked eye, they look unremarkable, but at the microscopic scale, they reveal a delicate dance between fluidity and structure. Their ability to mimic the mechanical properties of real tissue makes them especially valuable for medicine, from repairing injuries to 3D bioprinting functional tissue structures. Yet until now, one of the biggest challenges has been understanding exactly how these materials flow and deform when used inside the human body.
Unraveling the Mystery of Flow
At the center of this breakthrough are professors Brendan A. Harley and Simon A. Rogers, who joined forces to create a new framework that bridges material design and rheological modeling. Rheology—the science of how materials flow—may sound abstract, but in medicine, it is profoundly practical. If a hydrogel is too stiff, it cannot be injected. If it is too fluid, it won’t stay in place. The balance between these extremes determines whether a material can safely and effectively be used inside the body.
“For granular hydrogels to work,” Rogers explained, “you need to be able to put them inside of a body. This typically involves some sort of injection or printing process, which then means that we have to understand how these materials flow and deform.”
Past studies had attempted to measure this, but often in incomplete ways, missing the deeper physics that governs how these gels behave under stress. Rogers’ lab developed an advanced rheological model—the Kamani-Donley-Rogers model—that introduces a concept called brittility, a measure of where a material falls on the spectrum between ductile (stretchy and forgiving) and brittle (stiff and breakable). By combining this with yield stress, or the force needed to make a material start to flow, the researchers created a predictive tool that can accurately describe granular hydrogels under real-world conditions.
This new model doesn’t just describe what the gels do—it allows scientists to design them intentionally, tailoring their properties to fit the needs of specific tissues.
Engineering Materials for Life
For Harley, whose lab focuses on implantable biomaterials and tissue models, this framework opens entirely new doors. One of his major research interests is creating models of bone marrow, the soft tissue inside our bones where blood and immune cells are born. Healthy bone marrow is essential for human life, but as people age, its dynamics change, and the risk of diseases such as multiple myeloma increases.
“The ability to create and characterize increasingly sophisticated granular models of the bone marrow,” Harley explained, “is offering an entirely new way to understand how this evolution in properties over time affects how these essential cells behave.”
By integrating Rogers’ rheological insights with Harley’s biomaterials expertise, the team can now engineer hydrogels that closely mimic the unique properties of bone marrow, cartilage, or other tissues. This not only helps researchers understand disease progression but also provides a powerful platform for testing new therapies before they ever reach patients.
The Science of Collaboration
The partnership between Harley and Rogers highlights something profound: breakthroughs rarely happen in isolation. Rogers brings the tools of rheology, which uncover how materials behave under force, while Harley brings the knowledge of how to design biomaterials that interact with living systems. Together, they created a framework that neither could have achieved alone.
“We’re starting to see a fundamental shift in biomedicine,” Harley said, “where our communities are increasingly using engineered tissue models. That means we have to have a better understanding of how to create increasingly sophisticated, increasingly realistic tissue models.”
This is not just about improving lab experiments—it is about making sure that models of tissue can accurately reflect the human body, so that medical discoveries translate more effectively into real treatments.
Toward Healing and Beyond
The implications of this work stretch far beyond academic curiosity. By giving scientists a way to predict and control the behavior of granular hydrogels, the Illinois team is laying the groundwork for therapies that could transform lives. Imagine damaged cartilage in a knee being restored by an injection of hydrogel carrying living cells. Imagine using hydrogel-based models to study how cancer spreads through bone marrow, accelerating the search for cures. Imagine printing living tissues on demand for transplant, with properties precisely tuned to match the recipient’s own body.
“This level of understanding,” Rogers emphasized, “will allow us to design new materials that will make people healthier, faster—and help them stay healthier for the long term.”
Their vision is bold, but the science is solid. By distilling a complex material into a few key parameters, the Kamani-Donley-Rogers model transforms an overwhelming puzzle into something manageable, testable, and ultimately useful for medicine.
The Human Dimension of Discovery
What makes this story most compelling is not only the science but the human impact it promises. At its core, this research is about healing—about helping joints move without pain, about protecting bone marrow so it can keep producing the cells that sustain life, about giving doctors better tools to fight disease.
Every breakthrough in physics, chemistry, or engineering eventually comes down to human well-being. The granular hydrogel framework developed at Illinois is another step in that journey—a bridge between laboratory insight and lived experience, between equations on a page and healthier lives for millions.
Looking Ahead
The future of medicine is being shaped particle by particle, model by model, collaboration by collaboration. What Harley, Rogers, and their team have achieved is more than just a clever framework—it is a foundation for a new era of biomaterials.
Their work reminds us that science is not only about answering questions but about asking them in the right way. It is about refusing to accept incomplete explanations and daring to create new ones. And above all, it is about harnessing the deep laws of physics and engineering to serve something greater: the health and longevity of humanity.
The story of granular hydrogels is still unfolding, but one thing is clear. These tiny gel particles, packed tightly together, carry within them not just the mechanics of flow but the promise of healing. And thanks to this new framework, that promise is closer to becoming reality.
More information: Gunnar B. Thompson et al, Granular Hydrogels as Brittle Yield Stress Fluids, Advanced Materials (2025). DOI: 10.1002/adma.202503635