The world is in the midst of an energy revolution. From electric cars to renewable power grids, the demand for powerful, reliable, and sustainable energy storage systems has never been greater. Lithium-ion batteries, the workhorses of modern technology, power everything from smartphones to satellites. But they are reaching their limits. Their energy density—how much energy they can store per unit weight—is close to being maxed out.
Scientists have long looked to lithium-sulfur (Li-S) batteries as the next great leap. Sulfur is cheap, abundant, and theoretically capable of storing far more energy than the cobalt- and nickel-based compounds in today’s batteries. A Li-S battery could hold up to five times the energy of a lithium-ion battery of the same weight, transforming electric transportation and grid storage.
Yet there has been a persistent problem: Li-S batteries die too quickly. After just a few hundred charge and discharge cycles, they lose capacity, becoming unreliable. At the heart of this challenge lies a tricky chemical phenomenon involving sulfur compounds known as polysulfides.
Now, a groundbreaking study led by Prof. Yan Lu of Helmholtz-Zentrum Berlin (HZB) and Prof. Arne Thomas of the Technical University of Berlin may have changed the game. Their work has revealed a material that not only stabilizes lithium-sulfur batteries but also significantly extends their lifespan.
The Trouble with Polysulfides
To understand the breakthrough, we need to dive into the inner life of a Li-S battery. Inside, lithium ions move back and forth between the anode and cathode during charging and discharging. The cathode contains sulfur, which reacts with lithium to form lithium polysulfides (LiPSs).
Here’s the catch: polysulfides are restless. They dissolve into the electrolyte and drift through the battery like escape artists, breaking free from where they’re supposed to stay. This leads to two major problems:
- The battery loses active material, reducing its capacity.
- The “wandering” polysulfides react where they shouldn’t, creating instability and shortening the battery’s life.
For years, researchers have tried to trap these slippery polysulfides—like trying to hold water in a sieve. The idea was to create structures inside the cathode that could capture and bind the polysulfides, forcing them to react in place rather than escaping. Crystalline organic frameworks with sponge-like pores seemed promising, but the solutions were never stable enough. Until now.
A Radical New Material
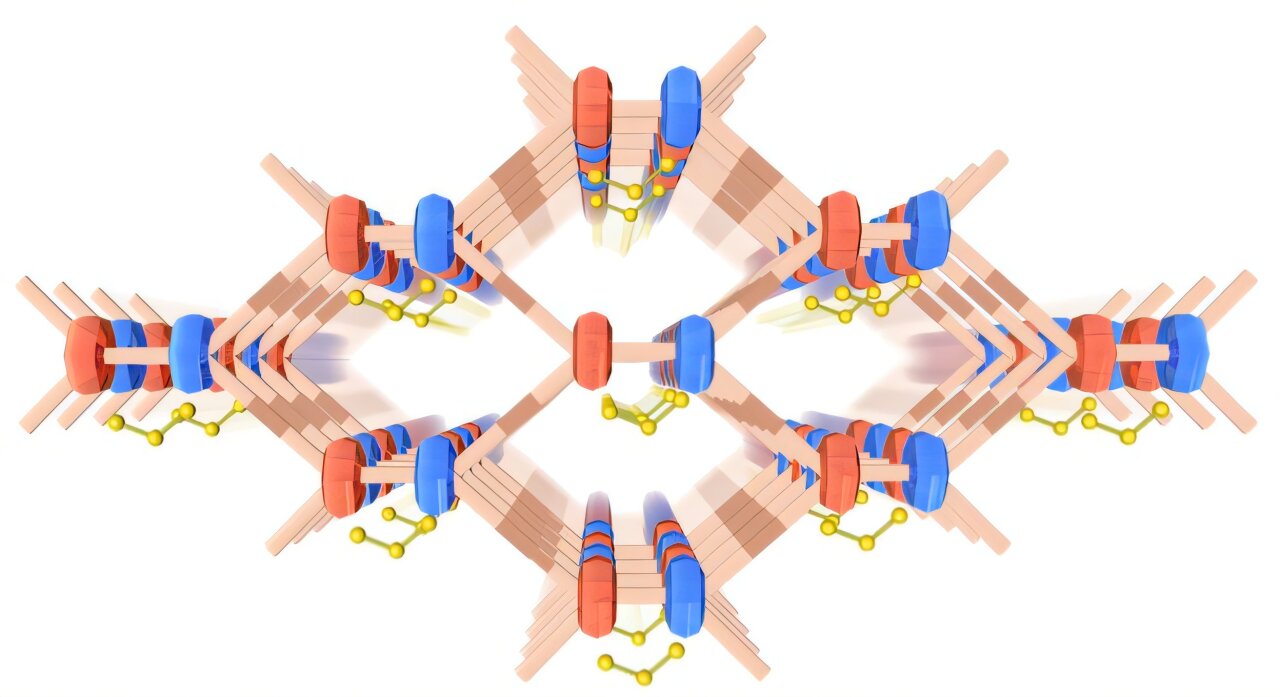
The Berlin team has introduced a fascinating material: a radical-cationic covalent organic framework (COF), built from polymers that form open, sponge-like pores on a microscopic scale. The key innovation lies in the framework’s chemistry.
At the heart of this new COF are tetrathiafulvalene units ([TTF]2•+) paired with trisulfide radical anions (S3•-), linked by benzothiazole. These radical structures carry unpaired electrons, which give them remarkable properties.
“Unpaired electrons play an important role in the micro/mesopores of COFs,” explains Prof. Lu. “They contribute to delocalized π orbitals, which facilitates charge transfer between the layers and thus improves the catalytic properties.”
In simple terms: the radical framework doesn’t just trap polysulfides—it actively catalyzes their reactions, making the process faster, more efficient, and more stable. The polysulfides are no longer troublesome fugitives but cooperative players in the battery’s chemical drama.
A Symphony of Experiments
Developing such a material required a careful blend of advanced experimental techniques. The team used solid-state nuclear magnetic resonance (ssNMR) spectroscopy to probe the chemical environment inside the COF. They employed electron spin resonance (EPR) spectroscopy to study the behavior of the unpaired electrons that give the material its radical character.
Crucially, they also turned to in situ X-ray tomography at the BAMline at BESSY II, a powerful synchrotron facility in Berlin. This allowed them to peer into the pores of the material and see, in real time, how its structure interacts with sulfur compounds. The experimental data was then combined with theoretical calculations, building a detailed picture of how the radicals catalyze sulfur reduction reactions.
Sijia Cao, a Ph.D. student on Prof. Lu’s team, describes the mechanism: “The radical cations [TTF]2•+ act as catalytic centers that bind LiPSs and facilitate the elongation and cleavage of the S–S bonds.” In other words, the radicals help guide the chemical transformations in a controlled, efficient way, rather than letting polysulfides run wild.
Record-Breaking Battery Performance
The payoff is astonishing. With the new radical COF—dubbed R-TTF•+-COF—lithium-sulfur batteries achieved a durability never seen before.
Where typical Li-S batteries begin to fail before 1,000 charge cycles, the new material allowed batteries to last for over 1,500 cycles, with an almost negligible capacity loss of only 0.027% per cycle.
This is not just an incremental improvement; it’s a leap. For the first time, Li-S batteries with COF-based materials show true long-term stability, moving them much closer to practical, commercial use.
Prof. Lu emphasizes the potential: “Integrating such radical scaffold structures into lithium-sulfur batteries shows great promise.” But he also stresses that the work is not finished. Different radical molecules may offer different catalytic strengths and electronic properties, opening the door to further optimization.
The Promise of Radical Frameworks
The implications of this breakthrough go beyond just one battery chemistry. The idea of building radical frameworks—materials with stable unpaired electrons in carefully engineered porous networks—could be applied to many areas of electrochemistry. These materials are like playgrounds for reactions: highly structured, highly active, and customizable.
In the case of Li-S batteries, they solve the polysulfide problem by turning chaos into order. But in other energy technologies, such as fuel cells or advanced supercapacitors, similar frameworks might guide different tricky reactions, opening entirely new frontiers.
Toward a Sustainable Future
The race for better batteries is not just about convenience—it’s about the future of our planet. As the world shifts away from fossil fuels, the ability to store renewable energy becomes critical. Wind and solar power are intermittent; they need robust, long-lasting storage to provide electricity when the sun isn’t shining and the wind isn’t blowing.
Lithium-sulfur batteries, with their high energy density and reliance on abundant sulfur rather than rare metals, could be a cornerstone of this future. Imagine electric cars that drive for 1,000 kilometers on a single charge, or grid storage systems that keep entire cities running overnight.
The discovery of radical COFs brings this vision closer to reality. It is a triumph not just of chemistry and physics, but of human ingenuity and persistence.
A Radical Leap Forward
Every great scientific breakthrough begins with a stubborn problem. For lithium-sulfur batteries, the wandering polysulfides were that problem. For years, they seemed unbeatable. Now, thanks to the radical frameworks developed by Prof. Lu, Prof. Thomas, and their team, the tide has turned.
The story is still unfolding, with much research yet to be done. But the message is clear: sometimes, the solution lies not in suppressing nature’s quirks, but in harnessing them. By embracing the strange power of radicals and weaving them into elegant porous frameworks, scientists have opened the door to a new era of battery technology.
The promise of longer-lasting, more powerful, and more sustainable batteries is no longer a distant dream—it is on the horizon.
More information: Sijia Cao et al, A Radical-Cationic Covalent Organic Framework to Accelerate Polysulfide Conversion for Long-Durable Lithium–Sulfur Batteries, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c09421