Life at the cellular level is a dynamic, constant balancing act between birth and death, creation and dissolution. For every new cell that springs into existence, another must be gently retired. This process, called apoptosis, is not a sign of failure, disease, or dysfunction—it is the elegant choreography of life itself. Apoptosis, often called programmed cell death, is the body’s way of sculpting, protecting, and renewing itself. It is a biological form of self-sacrifice, where cells choose to die for the greater good.
To the casual observer, death and life seem like opposing forces. But in the microscopic world, they are inextricably linked. Without apoptosis, the human body would be an uncontrollable mass of excess cells, riddled with malformations, disease, and unchecked growth. Apoptosis is not an accident. It is a finely tuned cellular symphony, performed with purpose, precision, and poetic grace.
A Lesson from the Embryo
The story of apoptosis begins in the earliest chapters of life. Imagine an embryo, not yet shaped into a human form, merely a cluster of multiplying cells. At this stage, everything is growth and expansion. But even as the body begins to take shape—fingers separating from hands, eyes forming from folds of skin—death is already at work.
One of the most visual examples of apoptosis is how it sculpts the fingers and toes. Early in development, the human hand is shaped like a paddle. Only through the programmed death of specific cells between the fingers do distinct digits emerge. Apoptosis is the invisible sculptor carving form out of formlessness.
And that is just the beginning. During the development of the nervous system, the brain produces far more neurons than it ultimately uses. Apoptosis prunes the excess, ensuring efficient wiring and functional circuitry. In a way, the cells that die are making room for clarity, focus, and connection. Without apoptosis, life would be malformed and chaotic.
The Mechanics of Dying with Dignity
At its heart, apoptosis is a tightly regulated biological process. Unlike necrosis—cell death due to injury or trauma—apoptosis is controlled, clean, and contained. It doesn’t spill its contents into surrounding tissue or provoke inflammation. Instead, a cell undergoing apoptosis dies in silence, dismantling itself from the inside out with surgical precision.
It begins with signals—either from inside the cell or from its environment—that activate the apoptotic pathway. These signals might arise due to DNA damage, lack of survival signals, or the need to eliminate cells no longer needed. Once triggered, the cell begins a cascade of molecular events.
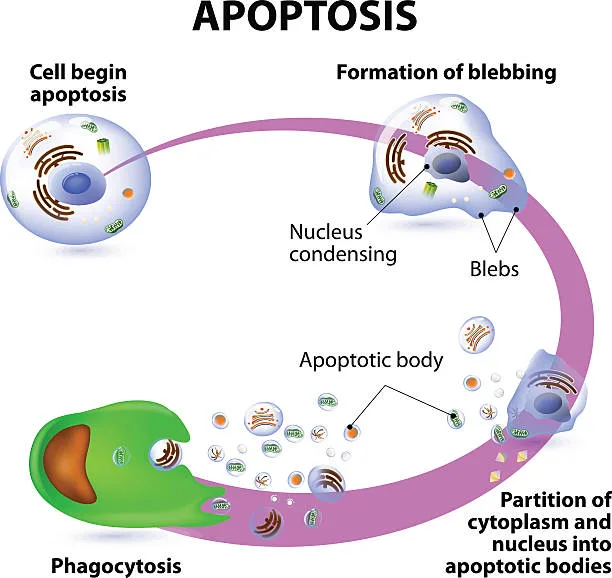
The nucleus condenses. The DNA fragments. The cell shrinks, detaches from its neighbors, and breaks into small, membrane-bound pieces called apoptotic bodies. These bodies are swiftly consumed by neighboring cells or specialized immune cells known as phagocytes. No trace remains. No inflammation occurs. It is, biologically speaking, the most respectful way to die.
The Caspase Orchestra: Executioners of Order
At the molecular level, apoptosis is orchestrated by a family of enzymes known as caspases—short for cysteine-aspartic proteases. These are the executioners of the cell, lying dormant until activated. Once the cell receives the death signal, initiator caspases (like caspase-8 or caspase-9) are switched on. They, in turn, activate effector caspases (like caspase-3 and caspase-7), which then begin dismantling the cell’s proteins and structural components.
Each caspase targets specific molecules. Some cleave nuclear lamins, which hold the nucleus together. Others cleave DNA repair enzymes, ensuring that no rescue operation will interfere with the dying process. This cascade, once set in motion, is irreversible. There’s no turning back. The cell is committed to its own demise.
What’s striking is the precision. There’s no chaos, no accidental damage to neighboring cells. Apoptosis is an internal implosion, guided by molecular design, ensuring the body continues to function with clarity and order.
The Pathways of Death: Intrinsic and Extrinsic
Apoptosis can be initiated in two major ways: from within the cell (the intrinsic pathway) or from signals outside the cell (the extrinsic pathway). Both converge on the caspase cascade, but their triggers differ.
The intrinsic pathway is triggered by internal stress—DNA damage, oxidative stress, or disruption in cell metabolism. This leads to the permeabilization of the mitochondria, the cell’s energy factories. Mitochondria release cytochrome c, a protein that then activates caspase-9 and begins the apoptotic cascade. The mitochondrion, often called the powerhouse of the cell, here becomes the harbinger of death—a quiet reminder that life and death dwell side by side even within a single organelle.
The extrinsic pathway, in contrast, is initiated by external signals. These come in the form of death ligands—proteins like FasL or tumor necrosis factor (TNF)—which bind to receptors on the cell surface. These receptors then recruit and activate initiator caspases, particularly caspase-8, beginning the cell’s dismantling.
This dual-path system ensures that the body can respond both to internal abnormalities and external threats. If a cell is too damaged to repair itself, it can initiate its own end. If a cell is infected or poses a threat to the organism, the immune system can send a death signal to eliminate it.
Apoptosis and the Immune System: Silent Guardians
Apoptosis is a cornerstone of the immune system. During development, the body generates millions of immune cells with random receptors. Some of these receptors will recognize the body’s own tissues—a recipe for autoimmune disaster. Apoptosis removes these potentially dangerous cells during a process known as negative selection.
Even in adulthood, apoptosis maintains immune balance. After an infection, the immune system expands its army of white blood cells. Once the threat has passed, apoptosis reduces the numbers back to baseline, preventing unnecessary inflammation or tissue damage.
Apoptosis also helps eliminate infected cells. Cytotoxic T cells, a type of white blood cell, recognize and bind to infected or cancerous cells, releasing molecules like perforin and granzymes. These trigger apoptosis from within, ensuring that the compromised cell dies without harming its neighbors.
Without apoptosis, the immune system would be like a battlefield with no cleanup crew—a dangerous accumulation of cells, some misguided, some no longer useful, all potentially harmful.
When Apoptosis Fails: Cancer and Other Diseases
Apoptosis is a guardian of health—but like any system, it can malfunction. When apoptosis is blocked or dysfunctional, the consequences are often dire.
Cancer is perhaps the clearest example. Cancer cells evade apoptosis, allowing them to survive despite genetic damage, evade immune detection, and proliferate uncontrollably. Mutations in key genes—like p53, often called the “guardian of the genome”—can prevent apoptosis from being activated, even when cells are severely damaged.
P53’s role is to sense cellular stress and decide whether the cell should repair itself or undergo apoptosis. When p53 is mutated, that decision-making process is lost. Cancer cells with broken p53 can accumulate mutations, resist therapy, and become more aggressive.
In some cancers, apoptotic pathways are blocked downstream, at the caspase level. Others overexpress survival proteins like Bcl-2, which prevent mitochondrial-mediated apoptosis. In every case, cancer hijacks the very systems meant to keep it in check.
But cancer is not the only disease of failed apoptosis. Neurodegenerative disorders like Alzheimer’s and Parkinson’s are also linked to dysfunctional apoptosis—but in the opposite direction. Here, too many cells die. Neurons, once lost, are not easily replaced. If apoptosis is overactive or misregulated, healthy brain cells can be caught in its crossfire.
Autoimmune diseases also stem from apoptosis gone awry. When self-reactive immune cells escape deletion during development, they can attack the body’s own tissues. In lupus, for example, defective clearance of apoptotic cells can expose hidden DNA and proteins to the immune system, triggering chronic inflammation.
Apoptosis, therefore, is a double-edged sword—its balance must be exquisitely maintained.
Therapies and Targets: Rewiring Death in Medicine
Because apoptosis plays such a central role in disease, it has become a prime target for therapy. Cancer researchers, in particular, have worked to develop drugs that restore apoptotic signaling in tumors.
One class of drugs targets the Bcl-2 family of proteins. These proteins regulate mitochondrial-mediated apoptosis. By blocking the function of anti-apoptotic members like Bcl-2, these drugs re-sensitize cancer cells to death. Venetoclax, a Bcl-2 inhibitor, has shown promising results in treating certain types of leukemia.
Other therapies aim to reactivate p53, the master regulator. Though challenging—because p53 mutations are diverse and complex—some experimental compounds can restore its function or mimic its effects.
In neurodegenerative diseases, the goal is different: to inhibit apoptosis. Protecting neurons from untimely death could slow the progression of diseases like ALS, Huntington’s, or Alzheimer’s. Experimental drugs that block caspases or other parts of the apoptotic pathway are being tested for these purposes.
Even viral infections can be managed by modulating apoptosis. Some viruses, like HIV or cytomegalovirus, manipulate host apoptosis to evade immune responses. Understanding these interactions could help design better antiviral treatments.
Apoptosis, once an abstract molecular curiosity, is now a frontier of therapeutic hope.
A Philosophical Reflection: Death as Design
There is something profoundly moving about apoptosis. It is a process by which a living thing, acting with foresight and control, chooses to die to protect the whole. Cells, lacking consciousness, are not making moral choices—but the poetry remains.
It challenges us to see death not as an enemy, but as a necessary counterbalance to life. Apoptosis reminds us that endings can be purposeful, that removal is sometimes a path to renewal, and that within every system—biological or societal—health depends on the timely exit of parts no longer serving the whole.
Apoptosis also invites reflection on individuality. Each cell is a separate entity, but its fate is never only its own. It is part of a greater body, a complex system, and sometimes, its most noble contribution is to disappear.
Conclusion: The Beauty in Letting Go
Apoptosis is not merely a biological footnote. It is a fundamental principle of life—a quiet, orderly, and meaningful death, occurring billions of times a day in every human body. It sculpts our features, protects our health, regulates our immune response, and even determines our vulnerability to disease.
Its failure can give rise to cancer, while its excess can hollow the brain. Its manipulation holds promise for future cures. Its very existence is a marvel of evolution—a system so refined, so precisely tuned, that it reveals the elegance of life’s design.
In understanding apoptosis, we understand something deeper: that in biology, as in life, sometimes the greatest act of preservation is knowing when to let go.