At the heart of all life lies a powerful rhythm—a cycle that repeats itself with breathtaking precision and purpose. This rhythm is not found in the beating of the heart or the tides of the ocean, but in the very fabric of life itself: the cell cycle. Every second, billions of cells in your body divide, grow, specialize, and sometimes die, all in a carefully orchestrated sequence that ensures life continues seamlessly.
The cell cycle is the symphony of cellular existence. It drives embryonic development, heals wounds, replaces old tissues, and maintains the balance of the living world. And yet, within its elegance lies danger. When the cycle falters, diseases such as cancer may arise, hijacking its machinery for malignant purposes.
Understanding the cell cycle is not merely a biological pursuit—it is a quest to comprehend life’s innermost workings. From the humblest bacterium to the most complex human neuron, this cycle is the unifying pattern, the shared breath of life across Earth.
The Eternal Circle of Cellular Life
Every cell originates from another cell. This deceptively simple truth, first declared by Rudolf Virchow in 1855, encapsulates the continuity of life. To maintain this chain, cells must duplicate their contents and divide precisely. The cell cycle is the name given to this sequence—a tightly regulated process that encompasses the birth, growth, division, and sometimes death of a cell.
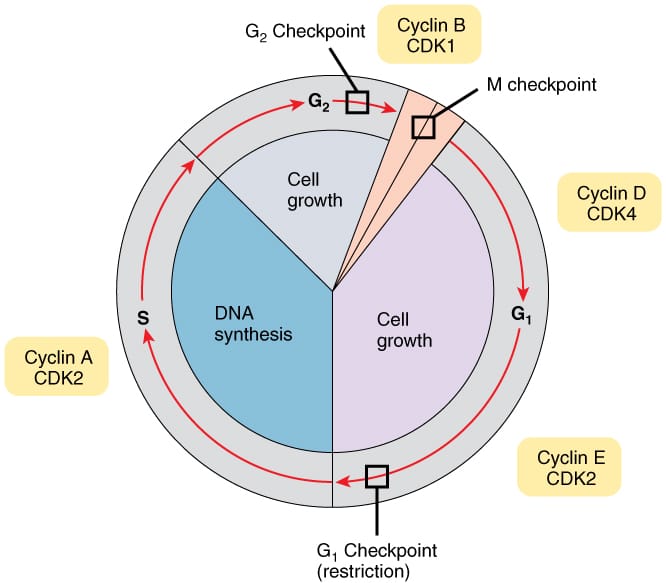
At its core, the cell cycle is divided into two major phases: interphase, where a cell prepares itself by growing and duplicating its DNA, and the mitotic phase, where the cell physically divides into two daughter cells. However, these stages are not isolated. They are part of a continuous flow—like the rise and fall of tides—with checkpoints ensuring that each step is completed faithfully before the next begins.
Cells in multicellular organisms don’t all divide constantly. Many remain in a quiescent phase, known as G0, performing their duties without proliferating. Others, like those in the skin, blood, or gut lining, cycle continuously, replacing lost or damaged cells. The decision to divide or remain idle is never random—it is dictated by internal needs and external signals, coordinated through molecular whispers and protein conversations.
Interphase: The Long Preparation
Interphase occupies most of the cell cycle—a time not of rest but of intense preparation. Within this span, a cell readies itself for the monumental task of dividing, and much of the action occurs silently beneath the microscope’s gaze.
The first phase within interphase is known as G1, short for “Gap 1.” Here, the cell grows, increases its supply of organelles, and builds the proteins it will need for DNA replication. It is a period of decision-making, a fork in the road. Depending on signals from the environment and the cell’s internal state, it may proceed, pause, or withdraw into the resting phase, G0.
If conditions are favorable, the cell transitions into the S phase—the synthesis phase. This is the stage of DNA replication. Every one of the cell’s chromosomes must be copied with absolute fidelity, ensuring that the resulting daughter cells inherit a complete and accurate genetic blueprint. The process is daunting: in human cells, over three billion base pairs of DNA are replicated in just a few hours, involving hundreds of proteins working in synchrony.
After replication comes G2, or “Gap 2,” where the cell checks the accuracy of DNA duplication and synthesizes the final proteins required for mitosis. It’s a stage of final inspection, as the cell ensures it has the tools, energy, and integrity to proceed to the climactic event of cell division.
Mitosis: The Dance of Division
Mitosis is the grand finale of the cell cycle—the moment when one cell becomes two. It is a feat of biological choreography, where every chromosome must be precisely divided and delivered to opposite ends of the cell. Errors are not merely undesirable—they can be catastrophic.
The process begins in prophase, where chromosomes condense into visible structures, and the nuclear envelope begins to break down. Each duplicated chromosome, consisting of two sister chromatids, is joined at a central point called the centromere. The mitotic spindle, a structure made of microtubules, begins to form. Its role will be to attach to the chromosomes and pull them apart with mechanical precision.
During metaphase, the chromosomes align at the center of the cell in a perfect line. This alignment ensures that each daughter cell will receive one copy of each chromosome. But alignment is not enough—the cell must confirm that every chromosome is correctly attached to spindle fibers before progressing.
Anaphase follows, marked by the sudden separation of sister chromatids. Like a curtain drawn back, they are pulled toward opposite poles of the cell. This movement is rapid, elegant, and tightly controlled.
Finally, in telophase, the chromosomes begin to decondense, and new nuclear envelopes form around each set. The cell has essentially formed two nuclei, each genetically identical to the original.
Mitosis ends with cytokinesis—the physical division of the cytoplasm into two daughter cells. In animal cells, a contractile ring pinches the cell in two, while in plant cells, a new cell wall forms between the daughter nuclei. The cycle is complete. Two new cells stand where one once existed, each ready to begin its own journey through the cycle.
The Guardians of the Cycle: Checkpoints and Control
A process so complex cannot be left to chance. Like traffic lights at intersections, checkpoints within the cell cycle ensure that each stage is completed accurately before the next begins. These checkpoints are the guardians of cellular integrity.
The first major checkpoint lies at the end of G1. Known as the G1/S checkpoint, it assesses whether the cell has adequate nutrients, energy, and undamaged DNA. If all conditions are favorable, the cell commits to DNA replication—a point of no return. This decision is governed by cyclins and cyclin-dependent kinases (CDKs), proteins that act as molecular switches. When activated, they trigger downstream events that propel the cell forward.
Another critical checkpoint exists at the G2/M boundary. Here, the cell verifies that DNA has been replicated fully and without error. If damage is detected, proteins like p53 halt the cycle and attempt repair. If repair fails, the cell may undergo apoptosis—programmed death—to prevent propagation of errors.
The final major checkpoint occurs during metaphase, ensuring that all chromosomes are correctly attached to spindle fibers. Known as the spindle assembly checkpoint, it prevents the cell from moving into anaphase until every chromosome is properly aligned. Failure here can lead to aneuploidy—a condition where cells have the wrong number of chromosomes, a hallmark of many cancers.
Molecular Maestros: Cyclins, CDKs, and p53
The cell cycle is governed not by a single conductor but by an ensemble of molecular maestros. Chief among them are cyclins and cyclin-dependent kinases (CDKs), proteins whose interactions control the progression through each phase.
Cyclins are so named because their levels rise and fall in a cyclical pattern. They bind to CDKs, activating them to phosphorylate other proteins—essentially flipping molecular switches that drive the cell forward. Different cyclins pair with different CDKs at different points in the cycle, creating a timed and coordinated series of activations.
Then there’s p53—a protein often called the “guardian of the genome.” When DNA damage occurs, p53 steps in to halt the cycle, initiate repair, or trigger cell death. Mutations in the gene that encodes p53 are found in more than half of all human cancers, highlighting its crucial role in maintaining genomic stability.
Other regulators include retinoblastoma protein (Rb), which suppresses the cell cycle until it is phosphorylated by CDKs, and ATM/ATR kinases, which sense DNA damage and activate repair pathways.
Together, these molecules form an intricate network of checks and balances—an internal logic that governs every decision a cell makes.
When the Cycle Goes Wrong: Cancer and Uncontrolled Division
The cell cycle is a marvel of precision. But when its regulation fails, the consequences can be devastating. Cancer is, at its core, a disease of the cell cycle—a breakdown in the control systems that normally limit growth and division.
Mutations in genes that encode cyclins, CDKs, or tumor suppressors like p53 or Rb can cause cells to divide uncontrollably. The result is a population of cells that grow without regard for the body’s needs, invading tissues, evading the immune system, and spreading throughout the body.
Many cancer therapies target the cell cycle. Chemotherapy and radiation often work by damaging DNA, pushing cancer cells past their tolerance for error and triggering apoptosis. Newer drugs target specific cyclins or CDKs, aiming to halt the cycle in cancer cells while sparing normal tissues.
Understanding the cell cycle is therefore not just an academic pursuit—it is the key to unlocking better treatments, earlier diagnoses, and perhaps one day, cures for humanity’s most persistent killer.
The Cell Cycle in Development and Healing
While cancer reveals the dark side of the cell cycle, its light side is no less powerful. During embryonic development, the cell cycle is a driving force of growth and differentiation. A single fertilized egg divides again and again, giving rise to the trillions of cells that make up a human being. During this time, the cycle operates at breakneck speed, with cells skipping G1 or G2 to replicate rapidly.
In adult life, the cycle remains essential for repair and regeneration. When you cut your skin, stem cells activate and re-enter the cycle to replace damaged tissue. In the liver, cells can regenerate large portions of tissue after injury. Even in the brain, once thought incapable of renewal, certain regions harbor stem cells that continue to cycle and create new neurons.
The cycle is also involved in aging. With each division, some cells gradually accumulate damage or shorten their telomeres—protective caps on chromosomes. Eventually, they reach a point called senescence, where they stop dividing but remain metabolically active. While senescence helps prevent cancer, its accumulation may contribute to aging and age-related diseases.
Checkpoints in Evolution: The Cycle Through Deep Time
The cell cycle is ancient—its core machinery conserved across eukaryotes from yeast to humans. This evolutionary conservation speaks to its fundamental importance. Yet it has also evolved flexibility, allowing different organisms and tissues to adapt it for specific needs.
Some organisms use variants of the cell cycle. Early embryonic divisions in frogs, for example, lack G1 and G2, allowing rapid development. Certain cells can exit the cycle permanently and become specialized—like red blood cells or neurons—while others can re-enter if needed.
Understanding how the cycle evolved sheds light on how complexity emerged in multicellular life. It also offers clues to why some animals regenerate better than others, or why some are more resistant to cancer. In the cell cycle, we glimpse not only life’s mechanics, but its history.
A Universe Within a Cell
The cell cycle is a story of elegance and endurance, of checks and balances, of growth and restraint. It is a silent clock ticking inside every living thing—a clock that does not just mark time, but creates it.
To study the cell cycle is to peer into the heart of life. It teaches us that order arises from chaos, that precision can emerge from complexity, and that even the smallest units of life are governed by laws as exacting as those of the cosmos.
As research continues to unveil its mysteries, the cell cycle stands as a testament to biology’s brilliance—a reminder that within every cell lies a world of wonder, turning, dividing, and renewing life with every beat.