Long before humans walked the Earth, before mammals, reptiles, or even fish swam through ancient oceans, microbes reigned supreme. These invisible architects of life—bacteria, archaea, fungi, and viruses—were the first to emerge on our young planet. They sculpted the atmosphere, created soil, broke down toxins, and built the molecular foundations for every living thing that followed.
Today, microbes are still with us, woven into our biology in ways that defy simple categorization. We often learn to fear them first: the germs that cause infections, the invisible enemies behind colds, food poisoning, and deadly diseases. But this is only half the story. Just as fire can destroy or sustain, microbes can be both harmful and essential.
In truth, we live in intimate partnership with trillions of bacteria—many of which are not enemies at all but our allies, protectors, and even co-architects of our bodies. This microbial duality—good vs. bad—forms the basis of one of the most important, misunderstood, and awe-inspiring relationships in biology: the dynamic between microbes and human health.
The Microbiome: Our Invisible Organ
The human body is home to an astonishing microbial ecosystem known as the microbiome. These communities exist in nearly every corner of the body: on the skin, in the lungs, within the mouth and nose, and especially in the gastrointestinal tract. The gut alone harbors a dense, diverse population of bacteria—estimated at over 100 trillion cells. That’s more than ten times the number of human cells in the body.
Far from being passive squatters, these microbes play crucial roles in digestion, immunity, metabolism, and even brain function. In fact, the microbiome is now considered by many scientists to function like an organ—an essential and active participant in the maintenance of human health.
Every person’s microbiome is unique, shaped by genetics, environment, diet, age, medications, and even birth method. Babies born vaginally acquire bacteria from their mother’s birth canal, while those born via C-section begin life with a different microbial signature, one that can influence immune development for years to come.
The symbiosis between humans and their microbes is ancient, complex, and remarkably cooperative. But it’s also delicate. Disruptions to this microbial harmony—whether from antibiotics, stress, poor diet, or illness—can tip the balance and transform once-helpful bacteria into dangerous adversaries.
Guardians of the Gut
One of the most remarkable examples of beneficial microbes at work can be found in the gut. Here, in the winding chambers of the small and large intestines, a bustling metropolis of microbes busily breaks down food, ferments fiber, and synthesizes essential nutrients. Certain gut bacteria produce short-chain fatty acids (SCFAs) that fuel the cells lining our intestines and regulate inflammation throughout the body.
These microbes also help us absorb vitamins like B12, K, and folate. Without them, our ability to digest complex carbohydrates—especially the plant fibers that make up a healthy diet—would be severely limited.
But perhaps even more important is their role as defenders. A healthy gut microbiome forms a living shield against invading pathogens. Beneficial bacteria compete with harmful microbes for space and resources, crowding them out and releasing antimicrobial compounds that kill or inhibit them. This microbial competition—called colonization resistance—is one of the first lines of defense against infection.
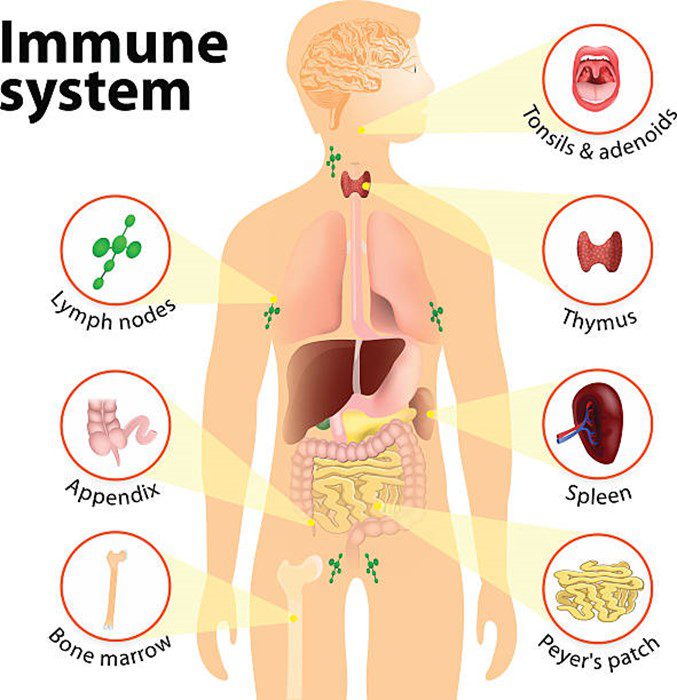
Some bacteria even “train” our immune system. From infancy, they help immune cells learn to distinguish between harmless substances and real threats. Without this microbial education, the immune system may overreact to non-dangerous stimuli, contributing to allergies, asthma, and autoimmune diseases.
When Allies Turn Against Us
Despite their many benefits, bacteria can turn rogue. Some strains are inherently pathogenic—designed by nature to infect and cause disease. Others become harmful only under certain conditions. What matters most is context.
Take Escherichia coli (E. coli), a familiar name often associated with food poisoning. Most strains of E. coli are harmless residents of the gut, quietly helping with digestion and vitamin production. But certain strains—like E. coli O157:H7—can cause severe, sometimes fatal illness by producing powerful toxins.
Similarly, Staphylococcus aureus lives harmlessly on the skin or in the nose of many people. But if it enters the bloodstream through a cut or surgical wound, it can cause life-threatening infections. When resistant to antibiotics, as in MRSA (methicillin-resistant Staphylococcus aureus), treatment becomes even more difficult.
Even beneficial bacteria can turn pathogenic if the balance of the microbiome is disturbed—a state known as dysbiosis. Factors like antibiotics, chemotherapy, stress, or a poor diet can wipe out beneficial microbes, giving opportunistic bacteria room to grow. The result can be gastrointestinal disorders, chronic inflammation, or infections such as Clostridioides difficile, a bacterium that thrives after antibiotic use and causes severe diarrhea and colitis.
The Double-Edged Sword of Antibiotics
Few discoveries have saved more lives than antibiotics. Since the first dose of penicillin was administered in the 1940s, antibiotics have revolutionized medicine, making once-deadly infections curable and enabling safe surgery, chemotherapy, and organ transplants.
But antibiotics are not selective. They kill bacteria indiscriminately, wiping out both harmful pathogens and beneficial microbes. A single course of broad-spectrum antibiotics can drastically alter the gut microbiome, sometimes for months. In some cases, microbial diversity never fully recovers.
This collateral damage has profound consequences. Antibiotic use, especially in early life, has been linked to increased risk of asthma, obesity, allergies, and autoimmune diseases. In older adults, it can impair digestion and weaken the immune response.
Overuse of antibiotics also contributes to one of the most serious public health threats of our time: antibiotic resistance. As bacteria evolve defenses against our drugs, common infections become harder—and sometimes impossible—to treat. In this microbial arms race, our past victories may sow the seeds of future defeats.
Probiotics and Prebiotics: Feeding Our Microbial Allies
In recent years, growing awareness of the microbiome’s importance has led to an explosion of interest in probiotics—live microorganisms that, when consumed in adequate amounts, confer health benefits. Found in fermented foods like yogurt, kefir, kimchi, and sauerkraut, as well as in supplements, probiotics aim to replenish or support beneficial bacteria in the gut.
Scientific evidence for probiotics is still evolving. Some strains have been shown to reduce the severity of diarrhea, prevent certain infections, or alleviate symptoms of irritable bowel syndrome. But not all probiotics are created equal. The benefits are often strain-specific, and some commercial products contain bacteria that are not effective or are present in insufficient quantities.
Prebiotics, on the other hand, are dietary fibers that nourish beneficial bacteria already living in the gut. Found in foods like onions, garlic, bananas, and asparagus, prebiotics encourage the growth of helpful microbes and enhance SCFA production. Together, probiotics and prebiotics offer promising strategies to support microbial health, though much remains to be understood.
Microbes and the Mind: The Gut-Brain Connection
Perhaps one of the most intriguing discoveries of the past two decades is the link between gut microbes and brain function—a connection so powerful it’s often called the gut-brain axis. The vagus nerve, immune system, and chemical signals produced by gut bacteria all contribute to this complex communication network.
Certain microbes can influence the production of neurotransmitters like serotonin, dopamine, and GABA, affecting mood, anxiety, and behavior. Studies have linked gut dysbiosis to depression, autism spectrum disorders, and even neurodegenerative diseases like Parkinson’s.
Animal experiments have shown that transplanting gut bacteria from anxious mice into calm mice can induce anxiety-like behavior. In humans, fecal microbiota transplantation (FMT)—a process in which gut bacteria from a healthy donor are introduced into a patient—has shown promise not just for treating infections like C. difficile, but for modulating mood and cognition.
While these findings are still emerging, they underscore the profound influence that microbes can exert far beyond the gut.
The Skin’s Microbial Shield
While the gut microbiome often gets the spotlight, the skin is another critical frontier. As our largest organ and the first barrier against the external world, the skin is home to a diverse array of bacteria, fungi, and viruses. These microbes form the skin microbiome, a community that helps regulate inflammation, prevent colonization by pathogens, and maintain the skin’s acidic pH.
Conditions like acne, eczema, and psoriasis are now believed to be influenced, in part, by imbalances in the skin microbiome. For example, the overgrowth of certain Cutibacterium species can trigger acne, while reduced microbial diversity is associated with inflammatory skin diseases.
Just as with the gut, excessive hygiene practices, antibiotic overuse, and harsh skincare products can damage the skin’s microbial balance. Ironically, in our pursuit of cleanliness, we may be making ourselves more vulnerable to the very infections we’re trying to avoid.
Birth, Breast Milk, and the Foundations of Microbial Health
Microbial health begins at birth. The method of delivery—vaginal birth or C-section—has a profound impact on a newborn’s microbial inheritance. Babies born vaginally acquire microbes from the mother’s birth canal and gut, while C-section babies are first colonized by skin and hospital bacteria.
Breastfeeding also plays a crucial role. Human breast milk is not sterile; it contains beneficial bacteria and specialized sugars called human milk oligosaccharides (HMOs) that are indigestible by infants but serve as food for gut microbes—especially Bifidobacteria, a genus critical for immune development and gut health.
These early microbial exposures influence everything from metabolism and immunity to brain development. Disruptions—such as early antibiotic exposure, formula feeding, or lack of microbial diversity—can increase the risk of allergies, asthma, obesity, and chronic inflammation later in life.
When Microbes Heal: Fecal Transplants and Microbiome Therapies
One of the most dramatic examples of microbial medicine is fecal microbiota transplantation (FMT). In this procedure, feces from a healthy donor—rich in beneficial bacteria—are transplanted into the gut of a patient suffering from dysbiosis. FMT has shown remarkable success in treating recurrent C. difficile infections, with cure rates exceeding 90% when antibiotics fail.
Microbiome-based therapies are also being explored for inflammatory bowel disease (IBD), ulcerative colitis, and even cancer. Some cancer treatments are more effective in patients with certain gut bacteria, suggesting that the microbiome could play a role in immunotherapy responses.
Research into microbiome “fingerprints” may one day allow personalized medicine approaches that tailor diets, drugs, and therapies to an individual’s unique microbial profile.
The Future of Human-Microbe Relationships
We are at the dawn of a microbial renaissance—a shift in perspective that sees microbes not just as threats but as collaborators in health and disease. Understanding and nurturing this relationship may hold the key to preventing and treating a host of modern ailments.
Yet, this future also depends on respecting the fragile balance of microbial life. It requires reducing unnecessary antibiotic use, encouraging diets rich in fiber and fermented foods, supporting diverse microbial exposures, and rethinking medical practices that sterilize more than they heal.
Technology will undoubtedly play a role—sequencing, synthetic biology, microbial engineering—but so will ancient wisdom: the idea that health is not the absence of disease, but the presence of harmony.
Conclusion: A New Way of Seeing Life
The story of microbes and human health is not a simple tale of good versus bad. It is a story of coexistence, co-evolution, and co-dependence. It is a reminder that we are not alone—not just in the cosmos, but in our own bodies. We are walking ecosystems, each of us a living mosaic of human and microbial life.
Embracing this truth means letting go of the fear-driven narratives that have long shaped our understanding of germs. It means recognizing that the bacteria on your skin, in your gut, and throughout your body are not invaders to be vanquished, but partners to be cherished.
In this dance of cells and signals, health is not a solitary achievement but a symphony—and microbes are part of the orchestra. By listening more carefully to their music, we may yet learn how to heal ourselves in deeper, more lasting ways.