Brain cancer has always been one of medicine’s most unforgiving foes. Among its many forms, glioblastoma stands as the most aggressive and devastating. Even with surgery, chemotherapy, and radiotherapy, survival rates remain painfully low—just 15% of patients live beyond five years after diagnosis. Families watch loved ones go through treatments that buy only a little more time, while scientists worldwide search desperately for new ways to fight back.
Now, researchers at the University of Cambridge may have discovered a game-changing approach—one that doesn’t attack cancer cells directly, but instead freezes the very environment that allows them to spread.
Their work, published in Royal Society Open Science, could mark the beginning of a new kind of therapy for brain cancer, offering hope where treatments have long fallen short.
The Role of Hyaluronic Acid: A Hidden Player in Cancer Spread
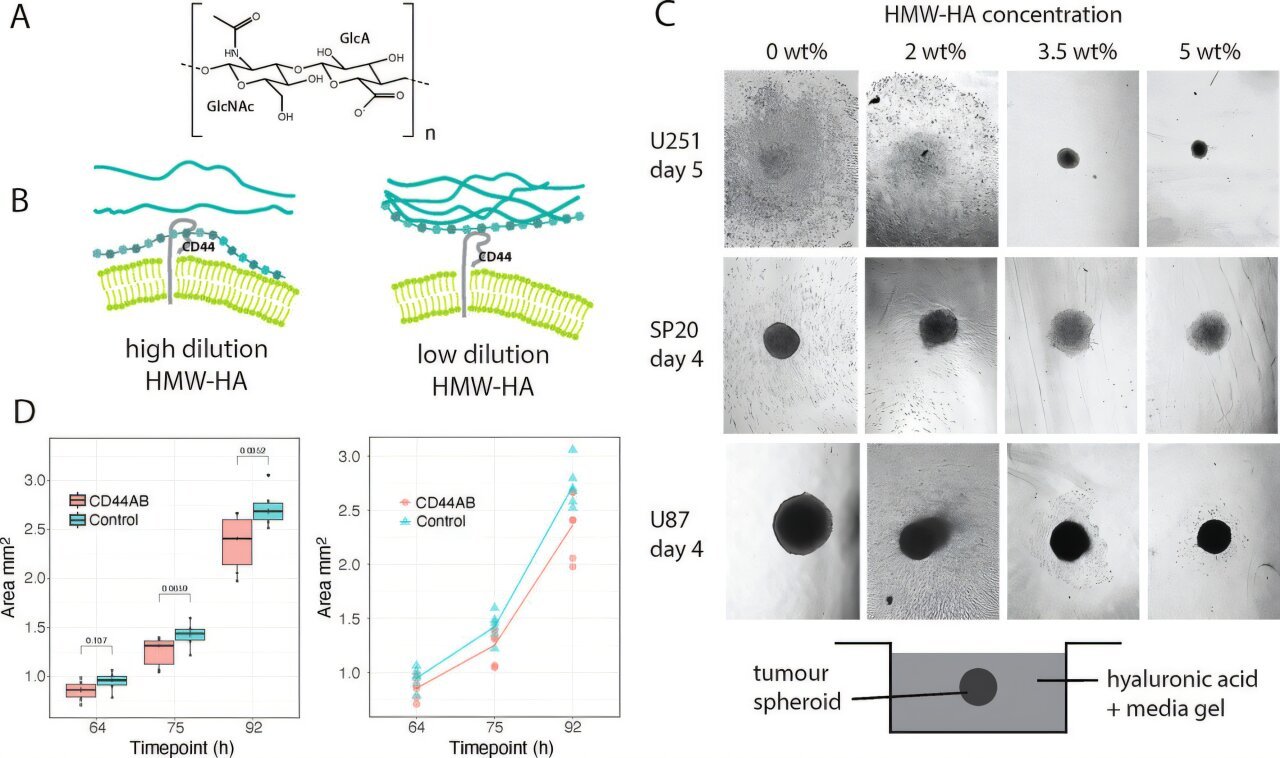
To understand this breakthrough, we need to look at a surprising molecule already abundant in the brain: hyaluronic acid (HA).
You may have heard of HA before—it’s often found in skincare products for its ability to retain moisture. But in the brain, HA plays a far more vital role. It makes up much of the brain’s supporting framework, known as the extracellular matrix.
Cancer cells, however, are clever opportunists. Glioblastoma cells exploit HA’s flexibility to latch onto a receptor called CD44 on their surface. This binding triggers the invasive behavior that allows cancer to spread through healthy brain tissue.
In other words, glioblastoma cells don’t just grow—they creep, using HA as a tool to slither their way into surrounding areas, making them nearly impossible to remove completely during surgery.
Freezing the Key That Unlocks Invasion
The Cambridge team, led by Professor Melinda Duer from the Yusuf Hamied Department of Chemistry, asked a bold question: What if we could stop HA from helping cancer cells move at all?
Using advanced techniques such as nuclear magnetic resonance (NMR) spectroscopy, the researchers discovered that HA molecules bend and twist into shapes that allow strong binding with CD44. But when they cross-linked the HA molecules—essentially “freezing” them in place—the binding signals were silenced.
The result was striking: glioblastoma cells simply stopped moving. They didn’t die, but they lost their ability to invade neighboring tissue.
“We didn’t have to kill the cells—we simply changed their environment,” Duer explained. “And when we did that, the cells gave up trying to escape.”
This represents a radical shift in cancer treatment strategy: instead of attacking the tumor directly, change the world around it so the cancer cannot thrive.
Why This Matters for Patients
Glioblastoma is notoriously resistant to traditional therapies. Even after surgeons remove as much of the tumor as possible, tiny cancerous cells left behind infiltrate healthy brain regions. Drugs often fail to penetrate the dense tumor tissue, and radiation can only slow, not stop, the disease.
This new approach could change that. By targeting the extracellular matrix rather than the cancer cells themselves, the treatment might prevent spread and recurrence—even when some tumor cells remain.
The research may also explain a troubling clinical mystery: why glioblastoma often returns at the site of surgery. After an operation, fluid build-up at the surgical site can dilute HA, making it more flexible and, ironically, creating an environment that encourages invasion. By freezing HA in place, recurrence might be slowed or even prevented.
“This could be a real opportunity to slow glioblastoma progression,” said Duer. “Because our approach doesn’t require drugs to enter every single cancer cell, it could in principle work for many solid tumors where the surrounding matrix drives invasion.”
A New Way of Thinking About Cancer
For decades, cancer treatment has focused on directly destroying tumor cells—cutting them out, poisoning them with drugs, or blasting them with radiation. But the Cambridge research highlights something fundamental: cancer cells don’t act alone. They are shaped by the environment around them.
If you can reprogram that environment, you can reprogram the cancer. This concept, known as matrix-based therapy, could represent a new frontier in oncology. Instead of killing cancer cells one by one, scientists may learn to shift the balance of their ecosystems so they become dormant, harmless, or even revert to normal behavior.
It’s a gentler, smarter approach—one that uses biology’s own rules to stop the disease in its tracks.
The Road Ahead
This discovery is still in its early days. For now, the effect has only been demonstrated in laboratory settings. The next crucial steps involve testing in animal models to ensure safety and effectiveness before moving to human clinical trials.
Many promising treatments stumble on this long road, but the concept is strong and the results so far are encouraging. If successful, this therapy could transform not just glioblastoma treatment, but potentially the treatment of other solid tumors that exploit their surrounding matrix.
For patients and families who have felt hopeless in the face of this aggressive disease, even the possibility of slowing progression is a source of new light.
Science and the Power of Changing Perspective
Science often advances not just through new discoveries, but through new ways of thinking. The Cambridge team’s breakthrough reminds us that sometimes the answer is not to fight harder, but to look differently.
By freezing a molecule most of us never think about, scientists may have found a way to freeze cancer’s spread. It’s a reminder that hope can emerge from the smallest details—and that the relentless pursuit of understanding may one day turn the tide against one of humanity’s deadliest cancers.
More information: Uliana Bashtanova et al, Molecular flexibility of hyaluronic acid has a profound effect on invasion of cancer cells, Royal Society Open Science (2025). DOI: 10.1098/rsos.251036