Every second of every day, your body is having conversations. But not in words. These conversations are chemical, silent, and swift, carried out by tiny molecules that race between cells with urgent messages: “Danger here,” “Heal this tissue,” “Call reinforcements,” “Stand down.” These molecules are cytokines—the unseen messengers of the immune system.
Cytokines are not just chemical signals. They are whispers, screams, and symphonies in the molecular language of immunity. They are the difference between life and death in an infection, the reason a cold clears up, and—when they go wrong—the storm that can cause sepsis or autoimmunity. To understand cytokines is to glimpse the secret workings of one of the most complex, delicate, and powerful systems in biology: the human immune response.
What Exactly Are Cytokines?
Cytokines are small proteins, typically made up of a few hundred amino acids, secreted by cells of the immune system and other cell types. Their job is to transmit signals from one cell to another. In essence, they are the molecular messengers that allow immune cells to communicate, coordinate, and execute defense strategies.
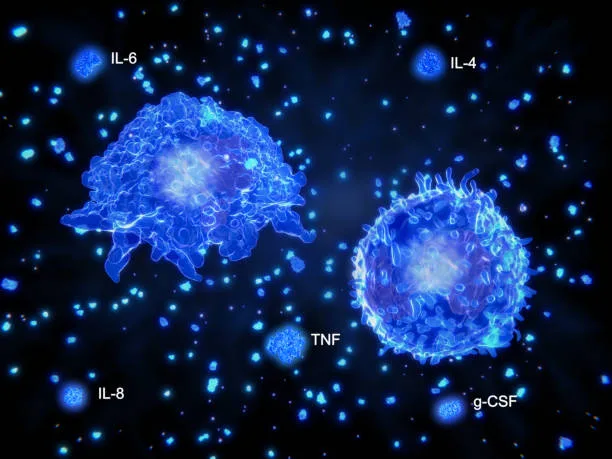
But cytokines are not a single entity. The term “cytokine” refers to a broad class of molecules, including interleukins, interferons, tumor necrosis factors, colony-stimulating factors, chemokines, and growth factors. Each type of cytokine has its own role, structure, and target. Some cytokines promote inflammation, others suppress it. Some help cells grow, others help them die. Some travel far, acting like long-distance dispatches; others stay close, like whispers between neighboring cells.
Though they are small, cytokines are extraordinarily powerful. In picogram amounts—trillionths of a gram—they can trigger sweeping changes in the behavior of cells, organs, and even the whole organism.
The Origins of Cytokine Signaling
The immune system didn’t always have cytokines. At least not as we know them. In the earliest life forms, immune responses were crude, nonspecific, and largely mechanical. But as evolution marched forward and multicellular life grew more sophisticated, a new need arose: communication. Cells had to talk to each other to coordinate responses, distinguish friend from foe, and remember past invaders.
Cytokines emerged as part of this molecular evolution. With them came a new level of immune sophistication. Innate immune cells like macrophages and dendritic cells could now call for help. Adaptive cells like T and B lymphocytes could coordinate strategies. Wounds could heal faster. Infections could be cleared more efficiently. Cytokines became the conductors of a cellular orchestra.
Over time, cytokine networks evolved to be astonishingly complex. The same cytokine might play entirely different roles depending on the context—who secretes it, who receives it, what else is happening in the tissue, and what receptors are available on surrounding cells. This context-dependence makes cytokines both wondrous and dangerous. The right signal at the wrong time can cause havoc. The wrong signal in the right place can lead to devastating disease.
The Cytokine Cascade: From Alarm to Action
Imagine a splinter piercing your finger. Almost instantly, cells near the wound sense damage and foreign intrusion. Resident macrophages and dendritic cells respond first, releasing a surge of early-warning cytokines like tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6). These cytokines set off a localized storm of inflammation.
Blood vessels dilate, becoming leakier. White blood cells roll to the margins, squeeze out of capillaries, and flood the site. The area turns red, swells, and heats up. Pain intensifies. All of this is orchestrated by cytokines—molecules too small to see, working in quantities too minute to measure without sensitive instruments.
Soon, a broader immune response is underway. Chemokines—cytokines that direct cell movement—guide neutrophils and monocytes to the wound. These cells devour pathogens, clean up debris, and release their own cytokines to call more reinforcements or dial back the inflammation when the job is done.
If a virus invades instead, different cytokines take the stage. Infected cells release interferons—aptly named because they “interfere” with viral replication. Interferons alert neighboring cells, helping them prepare defenses. Natural killer cells are summoned. T cells are primed. The entire immune system rallies in coordinated effort.
In every scenario—bacterial invasion, viral infection, tissue injury—the same principle holds: cytokines shape the response. They are not just footnotes in immunology textbooks. They are the conductors, regulators, and sometimes instigators of everything that follows.
The Receptor Connection: How Cells Listen
For cytokines to work, they need receivers—proteins on the surfaces of cells called cytokine receptors. Each cytokine binds to a specific receptor or group of receptors, much like a key fitting into a lock. When this binding occurs, it triggers a series of biochemical events inside the cell. This is cytokine signaling.
Cytokine receptors activate intracellular signaling cascades. One of the most well-studied is the JAK-STAT pathway (Janus kinase–signal transducer and activator of transcription). Here’s how it works: a cytokine binds its receptor; the receptor activates JAK proteins inside the cell; JAKs phosphorylate STAT proteins; STATs enter the nucleus and change which genes are turned on or off.
This signaling cascade is rapid and efficient, and it allows a single cytokine to influence hundreds of genes. Depending on the context, these genes might lead to cell division, migration, antibody production, or programmed cell death. In other words, cytokines can tell a cell to live, divide, move, attack, or even die.
The precision of this system is remarkable. Cells express only the receptors they need, and only when appropriate. This means only the right cells hear the message, and only at the right time. It’s a biochemical conversation in a crowded cellular world, whispered only to those meant to hear.
Inflammation and the Cytokine Symphony
At its best, inflammation is a powerful, lifesaving response. It isolates infection, eliminates pathogens, and sets the stage for healing. But inflammation must be tightly controlled. Too little, and infection can run rampant. Too much, and the body begins to destroy its own tissues.
Cytokines play a central role in this delicate balance. Pro-inflammatory cytokines like IL-1, IL-6, and TNF-α are crucial for initiating defense. But anti-inflammatory cytokines, like IL-10 and transforming growth factor-beta (TGF-β), are equally important for winding down the response.
The balance between these two classes of cytokines—activators and suppressors—is the key to resolving inflammation without collateral damage. When this balance is lost, disease ensues.
Chronic inflammation, fueled by persistent cytokine signaling, underlies a vast array of human illnesses—autoimmune diseases like rheumatoid arthritis and lupus, metabolic conditions like type 2 diabetes, and even cancers. In these conditions, cytokines become trapped in feedback loops, unable to shut off. The immune system attacks not an invader, but the self.
Understanding how cytokines orchestrate and sometimes sabotage inflammation is one of the central challenges of modern immunology. It’s also the key to unlocking better treatments for some of humanity’s most stubborn diseases.
Cytokine Storms: When the Orchestra Goes Mad
In 2020, as the world struggled to understand a new and deadly coronavirus, one term leapt into the headlines: cytokine storm.
In severe cases of COVID-19, patients did not die solely from the virus itself, but from the body’s uncontrolled immune response. A massive surge of cytokines—particularly IL-6 and TNF-α—flooded the lungs, causing widespread inflammation, tissue damage, and multi-organ failure. This runaway reaction, known as a cytokine storm, is an immune system gone berserk.
Cytokine storms are not unique to COVID-19. They have been seen in influenza, Ebola, sepsis, and even in some cancer immunotherapies. In each case, the very molecules designed to protect the body turn destructive when released in excess.
The challenge in treating cytokine storms is finding a way to suppress the damaging inflammation without paralyzing the entire immune response. Drugs that block IL-6 receptors or TNF-α are among the tools being explored, but timing is everything. Intervene too late, and the damage is done. Suppress too much, and the virus gains the upper hand.
These storms remind us that cytokines are double-edged swords. They are essential for defense—but their power must be wielded with surgical precision.
Cytokines and the Future of Medicine
Cytokines are not just molecules of disease; they are also molecules of healing. In recent years, medical science has learned to harness cytokines as therapies.
In cancer treatment, synthetic cytokines like IL-2 and interferons are used to boost immune responses. In autoimmune disease, cytokine blockers help rein in the overactive immune system. Biologic drugs that block TNF-α, for example, have transformed the lives of patients with Crohn’s disease, psoriasis, and rheumatoid arthritis.
Gene therapies are being developed to deliver cytokines directly into tumors, turning cold cancers into hot ones—visible and vulnerable to immune attack. Meanwhile, CAR T-cell therapies, which engineer a patient’s own immune cells to fight cancer, depend on cytokines for expansion and function.
Even in vaccines, cytokines play a key role. Adjuvants—substances added to enhance immune responses—often work by triggering cytokine production. These molecules help shape memory and durability, ensuring the body remembers how to fight long after the vaccine is delivered.
Cytokines also offer hope in regenerative medicine. By guiding inflammation and promoting repair, cytokine therapies may help regenerate damaged tissue after stroke, heart attack, or spinal cord injury.
The future of medicine is molecular, and cytokines are among the brightest stars in that universe.
The Emotional Body: Can Cytokines Affect the Mind?
As scientists have learned more about cytokines, a strange and profound discovery has emerged: the immune system talks to the brain.
Cytokines can cross the blood-brain barrier or influence it from the periphery. When they do, they can alter mood, cognition, and behavior. The flu-induced lethargy, the depression that accompanies chronic inflammation, the brain fog after infection—all of these are, at least in part, mediated by cytokines.
Some researchers now believe that cytokines may play a role in psychiatric disorders. Elevated inflammatory cytokines have been found in depression, schizophrenia, and autism spectrum disorders. Clinical trials are exploring whether anti-inflammatory drugs can alleviate symptoms.
This area, known as psychoneuroimmunology, is young and complex, but it underscores an important truth: the immune system is not separate from the self. Its molecules can touch not only our tissues but our consciousness.
Conclusion: Listening to the Body’s Smallest Voices
Cytokines are not just proteins. They are voices in the biological chorus of life. They warn. They summon. They soothe. They rage. They are as subtle as a whisper and as catastrophic as a scream.
To understand cytokines is to listen—truly listen—to the body as it speaks in its own language. A language of chemistry, electricity, and feedback loops. A language that tells the story of survival.
In every infection fought, every wound healed, every vaccine that protects us, and every disease that challenges us, cytokines are there. Not seen, not felt, but orchestrating the grand, invisible ballet that keeps us alive.
And so, the next time your body mounts a fever, or your skin swells after a scrape, or you recover from a cold, remember this: somewhere deep inside, molecules no bigger than a virus were having a conversation. And that conversation may have saved your life.