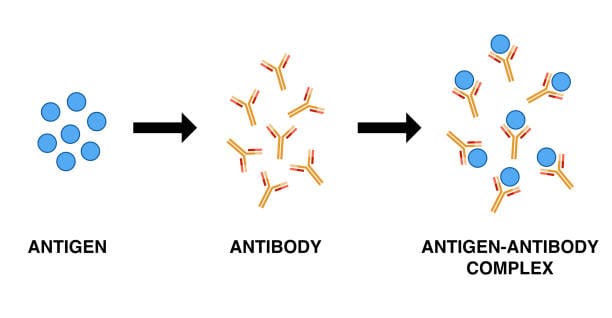
Imagine a vast and invisible battlefield within your body—one that is constantly under threat from millions of invaders that want to hijack your cells, steal your energy, or kill you slowly and silently. You don’t see this war, you don’t feel most of its skirmishes, and yet your survival depends entirely on its outcome. This battlefield is your immune system, and at the very heart of it are two of the most crucial players in the entire drama of life: antigens and antibodies.
To understand what keeps us healthy, what makes us sick, and how vaccines and immune therapies work, we must journey into the molecular world where antigens provoke a response and antibodies rise to fight. These two entities are locked in an evolutionary dance that has shaped not just human survival, but the history of all life on Earth. Understanding them is to peer into the elegant, brutal, and awe-inspiring mechanics of immunity itself.
The Identity Tags of Life
Let’s begin with antigens, the strange yet familiar villains—or sometimes just messengers—of this internal war. The term “antigen” is short for antibody generator, which offers the first clue about its purpose. Antigens are molecular structures, often proteins or polysaccharides, that are found on the surface of pathogens such as viruses, bacteria, and fungi. But they can also be found on cancer cells, toxins, transplanted organs, and even pollen or food in cases of allergies.
An antigen’s defining feature is that it is recognized by the immune system. It is like a badge, a unique pattern on the surface of a particle that screams either “self” or “enemy.” Every living cell wears these badges—markers made of proteins and sugars—on its surface. Your own cells are covered with “self” antigens that your immune system learns to ignore, unless something goes terribly wrong, as in autoimmune diseases.
Foreign antigens, however, trigger alarms. They are the uninvited guests at a party, wearing strange uniforms and speaking in unfamiliar chemical tongues. When these foreign shapes are detected, the immune system shifts into high alert. It is not the size of the invader that matters, but its identity—and antigens are how that identity is determined.
What makes antigens remarkable is their diversity. The surfaces of viruses like HIV or SARS-CoV-2 are dotted with spike proteins that act as potent antigens. Bacteria, in turn, might present flagella, cell wall components, or exotoxins—all recognizable as foreign. Even something as harmless as a peanut protein can act as an antigen if a person’s immune system mistakenly identifies it as dangerous. The immune system’s accuracy is generally incredible, but it is not perfect. And those imperfections, though rare, can lead to devastating consequences.
Antibodies: Nature’s Precision Weapons
In response to the presence of antigens, the immune system launches one of its most brilliant creations: the antibody. These are Y-shaped protein molecules produced by specialized white blood cells known as B lymphocytes, or simply B cells. When a B cell encounters an antigen, it goes through a process of activation and transformation, turning into a plasma cell capable of producing thousands of antibodies per second.
An antibody is a molecular missile—targeted, precise, and astonishingly specific. Its tips are engineered to bind to a particular antigen with exquisite accuracy, like a key fitting into a lock. This interaction is not casual. It is the core of immune recognition, the handshake that can either mean peace or war.
There are five major classes of antibodies in humans—IgG, IgA, IgM, IgE, and IgD—each with specialized roles. Some circulate in the bloodstream, hunting for pathogens. Others protect mucosal surfaces like the lungs or intestines. IgE is notorious for its role in allergic reactions. IgG, the most abundant, is a workhorse responsible for long-term immunity.
When antibodies bind to antigens, they do more than just mark them. They neutralize viruses by blocking their entry into cells, flag bacteria for destruction by phagocytes, activate the complement system to create pores in microbial membranes, and clump pathogens together in a process called agglutination, making them easier to eliminate.
But perhaps most profound is the memory that comes with antibodies. After the battle is won, memory B cells linger, sometimes for years or decades. Should the same antigen return, the immune system reacts faster, stronger, and more efficiently—a principle that makes vaccination one of the greatest achievements in medical science.
The Symphony of Immune Recognition
The elegance of the immune system lies in its selectivity. How can it distinguish between your own body and a threat? Between a helpful bacterium in your gut and a deadly strain of Salmonella? It comes down to recognizing antigenic patterns, and developing antibodies that match them.
Each B cell carries a unique receptor that can bind a specific antigen. Before they even encounter a threat, the human body contains around 10 billion different B cells, each one equipped to respond to a different possible invader. This staggering diversity is generated not by having 10 billion genes, but through a process of genetic shuffling and mutation that occurs during B cell development. It’s evolution, sped up and focused on one goal: the anticipation of an unknown future.
When a B cell’s receptor binds to an antigen, it’s a moment of destiny. That cell is activated, proliferates, and floods the system with matching antibodies. Meanwhile, T cells—another class of lymphocytes—help by presenting antigens, releasing signaling molecules, and killing infected cells directly. Together, they coordinate a response that is both systemic and specific.
This process is dynamic and adaptive. It learns. It changes. It builds memory. And it does all this without a central command, relying instead on networks of interactions, feedback loops, and chemical messengers. It’s not just a defense mechanism—it’s a biological intelligence.
Antigens in Vaccines: Teaching Without Disease
The concept of vaccination is based entirely on teaching the immune system to recognize antigens before a real infection occurs. By introducing a harmless version of a pathogen—or just part of it—the immune system is tricked into mounting a response. It creates memory cells and antibodies as if the threat were real, so that when the real pathogen arrives, the body is already prepared.
Traditional vaccines used weakened or inactivated viruses, like the polio or measles vaccines. More modern approaches, such as mRNA vaccines (like those developed for COVID-19), instruct cells to temporarily produce a piece of a viral protein—an antigen—without ever exposing the body to the virus itself.
In every case, the goal is the same: expose the immune system to a foreign antigen and provoke a response without causing disease. It’s like a fire drill for your cells. And when the day of reckoning comes, your immune system responds not with panic, but with precision.
When Things Go Wrong: Allergies and Autoimmunity
Antigens are not always dangerous, and antibodies are not always beneficial. When the immune system overreacts to harmless antigens—like pollen, pet dander, or food proteins—it results in allergic reactions. In these cases, antibodies (especially IgE) trigger the release of histamine and other inflammatory substances that cause symptoms like sneezing, itching, or anaphylaxis. The body treats an innocent substance as a deadly invader, unleashing a powerful and unnecessary response.
Even more tragically, the immune system can sometimes misidentify the body’s own cells as foreign. This is the basis of autoimmune diseases, such as lupus, rheumatoid arthritis, or type 1 diabetes. Here, antibodies target the body’s own tissues, leading to chronic inflammation and tissue destruction. It’s as if the security system has turned on the inhabitants.
Scientists still don’t fully understand why autoimmunity happens in some individuals and not others. Genetics, infections, environmental factors, and even gut bacteria may play a role. The immune system’s greatest strength—its ability to learn and adapt—can also be its greatest vulnerability.
The Science of Detection: Antibodies in the Lab
Beyond their natural roles, antibodies have become essential tools in biomedical science. In laboratories around the world, they are used in diagnostics, research, and treatment. Tests like ELISA and Western blotting rely on antibodies to detect the presence of specific proteins. Rapid tests for viruses, such as HIV or COVID-19, work by identifying antibodies or antigens in blood or saliva.
In cancer research, monoclonal antibodies—lab-created versions of antibodies that bind to one specific antigen—are used to target tumor cells. Drugs like Herceptin and Rituximab have revolutionized treatment by directing the immune system to fight cancer more effectively and precisely.
Antibody engineering is also the foundation of immunotherapy, a fast-growing field that harnesses the power of the immune system to treat disease. By training antibodies to seek out and destroy specific antigens, scientists are turning the immune system into a personalized weapon against previously untreatable conditions.
Antigens and Antibodies in Evolution
Long before humans appeared, life forms were already engaged in microscopic warfare. The struggle between host organisms and pathogens has been a major driver of evolution. Antigens evolve to escape detection. Antibodies evolve to catch up. This biological arms race has shaped everything from the diversity of immune genes to the structure of our genomes.
In fact, one of the most variable regions in the human genome is the major histocompatibility complex (MHC), which helps present antigens to immune cells. The MHC varies so widely among individuals that it makes organ transplantation extremely difficult—unless the donor and recipient are genetically similar.
This variability has a deep evolutionary purpose. Populations with a wide variety of antigen-recognition systems are more likely to survive pandemics. Diversity isn’t just a social ideal—it’s a biological necessity.
A Future Guided by Immune Precision
As we enter a new age of medical science, our understanding of antigens and antibodies is guiding innovation. Personalized vaccines for cancer, antibody-based drugs for chronic diseases, and even synthetic biology tools that design new immune responses are all based on these two molecular characters.
But we are also learning to tread carefully. The immune system is powerful but delicate. It can be trained, redirected, and amplified—but it can also be misled, overdriven, or sabotaged. The goal is not just to fight harder, but to fight smarter. To understand the language of antigens more clearly, and to craft antibodies that heal instead of harm.
From pandemics to autoimmune diseases, from allergies to cancer, the immune system is the stage on which our most critical biological battles are fought. And antigens and antibodies remain its most iconic players—each one a molecular echo of a much larger story.
The Immune Story Within Us
In the end, antigens and antibodies are not abstract scientific terms. They are part of us—of our biology, our vulnerability, and our resilience. They represent the primal and eternal dance between self and other, between danger and safety, between survival and collapse.
Every breath we take, every wound we heal, every virus we defeat silently involves the choreography of these molecules. They are writing the story of our lives in a language so ancient and elegant that we have only begun to understand its grammar.
So the next time you recover from a cold, get vaccinated, or read about a new medical breakthrough, remember this: the war was fought in silence, but the victory was written in antibodies. And the memory of that triumph will remain encoded within you—waiting, watching, ready.