Every second of every day, without our awareness, a vast and ancient army wages war inside our bodies. It is not a war we see or feel—until we become sick—but a relentless, microscopic struggle that keeps us alive. This army is the human immune system, and it is one of the most intricate, adaptable, and awe-inspiring creations of biological evolution. At the heart of this ceaseless defense is a singular and vitally important function: the ability to detect threats—viruses, bacteria, parasites, and anything that doesn’t belong. Detection is the frontline of survival. Without it, we would perish within days in a world teeming with microscopic enemies.
But how exactly does the immune system detect invaders? How does it distinguish friend from foe, self from not-self, harmless from harmful? To understand this, we must enter a universe more alien than outer space—a world of cells, proteins, and chemical signals, where identities are determined not by appearance but by molecular fingerprints. It is a realm of pattern recognition, surveillance, and response—a biological security system so fine-tuned that a single foreign molecule can trigger a massive immune reaction.
This is the story of how your body knows it’s under attack—and how it launches a defense that can be both miraculous and deadly.
The Body’s Need to Tell “Self” From “Other”
Long before any immune cell fires the biochemical equivalent of a gunshot, it must first ask a critical question: Is this part of me?
The human body is made up of trillions of cells, all bearing markers on their surfaces that identify them as “self.” These markers are like molecular name tags made of proteins and sugars. They’re called Major Histocompatibility Complexes, or MHC molecules, and they play a starring role in immune detection.
Think of MHC molecules as the body’s internal ID system. Every cell (with a few exceptions like red blood cells) wears them. When immune cells patrol the body, they’re constantly scanning these IDs. If a cell has the correct MHC markers, it is typically left alone. If the ID is missing, incorrect, or altered in any way, it may be flagged for destruction.
The ability to recognize “self” isn’t something cells are born with. It’s a learned behavior. During development in the thymus, a gland near the heart, T cells undergo a brutal selection process where they are tested against the body’s own proteins. Any T cell that reacts strongly to self is destroyed. Only those that ignore self—but are ready to react to the unfamiliar—are allowed to mature. This training is the first step in ensuring that the immune system can detect outsiders while sparing its own tissues.
Yet this process is not perfect. In autoimmune diseases like lupus or type 1 diabetes, the immune system mistakenly identifies parts of the body as invaders. The same machinery that saves us daily can sometimes turn on us, with devastating consequences.
Pattern Recognition: The Body’s First Alarm System
Imagine you’re a border patrol agent. You can’t possibly memorize the face of every potential enemy. Instead, you look for suspicious behaviors, strange clothing, or known symbols that mark someone as dangerous. That’s exactly what the innate immune system does.
The innate immune system is the body’s first line of defense. It is ancient, fast, and generalized. Its cells—such as macrophages, neutrophils, and dendritic cells—are equipped with special receptors called Pattern Recognition Receptors (PRRs). These PRRs are programmed to detect Pathogen-Associated Molecular Patterns (PAMPs)—molecular structures that are common in microbes but absent in human cells.
PAMPs include things like bacterial cell wall components (such as lipopolysaccharides), viral double-stranded RNA, or flagellin (a protein used by bacteria to swim). These molecules are evolutionary giveaways—signs that something foreign and potentially dangerous has entered the body.
When PRRs on immune cells recognize a PAMP, they trigger an alarm. This sets off a cascade of chemical signals—particularly the release of cytokines—that alert other immune cells, initiate inflammation, and call reinforcements to the site of infection. Blood vessels dilate. White blood cells pour in. The battlefield begins to form.
Among the most famous PRRs are the Toll-like receptors (TLRs). First discovered in fruit flies and named after a German word for “amazing” or “weird,” TLRs are now known to be essential for detecting many types of microbial invaders. Their activation is like throwing a switch that lights up the immune system.
Yet this system is only part of the story. It is the innate immune system’s job to buy time—to contain the invader until the more specialized, more powerful adaptive immune system can be activated.
The Adaptive Immune System: Molecular Intelligence at Work
While the innate system acts quickly, it is blunt and non-specific. The adaptive immune system, on the other hand, is slow to respond but incredibly precise. It is the part of the immune system that can learn, remember, and mount devastatingly specific attacks against threats it has seen before. It is also what makes vaccines possible.
Detection in the adaptive immune system depends on an entirely different strategy. Instead of scanning for patterns common to many microbes, adaptive immune cells—particularly B cells and T cells—carry receptors that are randomly generated to recognize a vast universe of possible molecular shapes, known as antigens.
An antigen is any piece of a molecule (usually a protein or carbohydrate) that can be recognized by an antibody or T cell receptor. The incredible thing is that B and T cells don’t wait to encounter a pathogen to develop their receptors. Instead, during development, they shuffle their DNA like a deck of cards, creating millions of unique receptors capable of binding to almost any shape in the universe.
When a B or T cell happens to encounter an antigen that matches its receptor—like a lock meeting its perfect key—it activates, divides, and launches an immune response. Some of these cells become memory cells, ready to respond faster and stronger if the same invader returns.
It’s a breathtaking system—one that combines randomness with specificity, learning with precision. It’s the biological equivalent of building an army in advance, each soldier holding a key that might one day match a lock in a future battle.
How T Cells Recognize Infected Cells
T cells are the assassins of the immune world, and they rely on a highly refined form of detection. There are two major types: helper T cells (CD4+) and cytotoxic T cells (CD8+). Both detect invaders in collaboration with MHC molecules.
While MHC markers normally present self-proteins, when a cell is infected by a virus or certain bacteria, it begins to display fragments of the invader—tiny peptides—on its MHC molecules. This is like waving a red flag.
Helper T cells scan peptides presented by MHC class II molecules, which are found on professional antigen-presenting cells like dendritic cells and macrophages. When they recognize a foreign peptide, they activate and release cytokines that stimulate other immune cells.
Cytotoxic T cells, on the other hand, patrol cells displaying MHC class I molecules, which are present on almost every cell in the body. If a cytotoxic T cell sees a virus-derived peptide on a cell’s MHC I, it recognizes that the cell is infected—and kills it. It releases enzymes that punch holes in the target cell’s membrane, triggering programmed cell death.
This deadly precision ensures that infected cells are destroyed before they can become virus factories, but it also underscores the importance of accurate detection. A T cell that misfires can kill innocent cells. The immune system must walk a tightrope between defense and destruction.
B Cells and the Marvel of Antibodies
If T cells are assassins, B cells are weapon designers. Their job is to produce antibodies—Y-shaped proteins that can recognize and bind to specific antigens with incredible precision.
Each B cell is unique. It carries a receptor on its surface that is a membrane-bound form of the antibody it can produce. When this receptor binds to an antigen—say, a spike protein on a virus—it gets activated (often with help from a helper T cell) and begins dividing. Some of the daughter cells become plasma cells, which churn out thousands of copies of the antibody.
Antibodies serve many roles in detection and defense. They can neutralize viruses by binding to them and blocking their ability to enter cells. They can tag bacteria for destruction by other immune cells—a process called opsonization. They can even activate the complement system, a cascade of proteins that punches holes in invaders.
What’s most astonishing is that antibodies don’t just fight—they remember. Once an infection has passed, some B cells become memory B cells. These cells lie dormant, ready to launch a rapid and potent response if the same pathogen returns. This memory is why vaccines work: they train the immune system to detect a pathogen it hasn’t seen—so that it’s ready when it does.
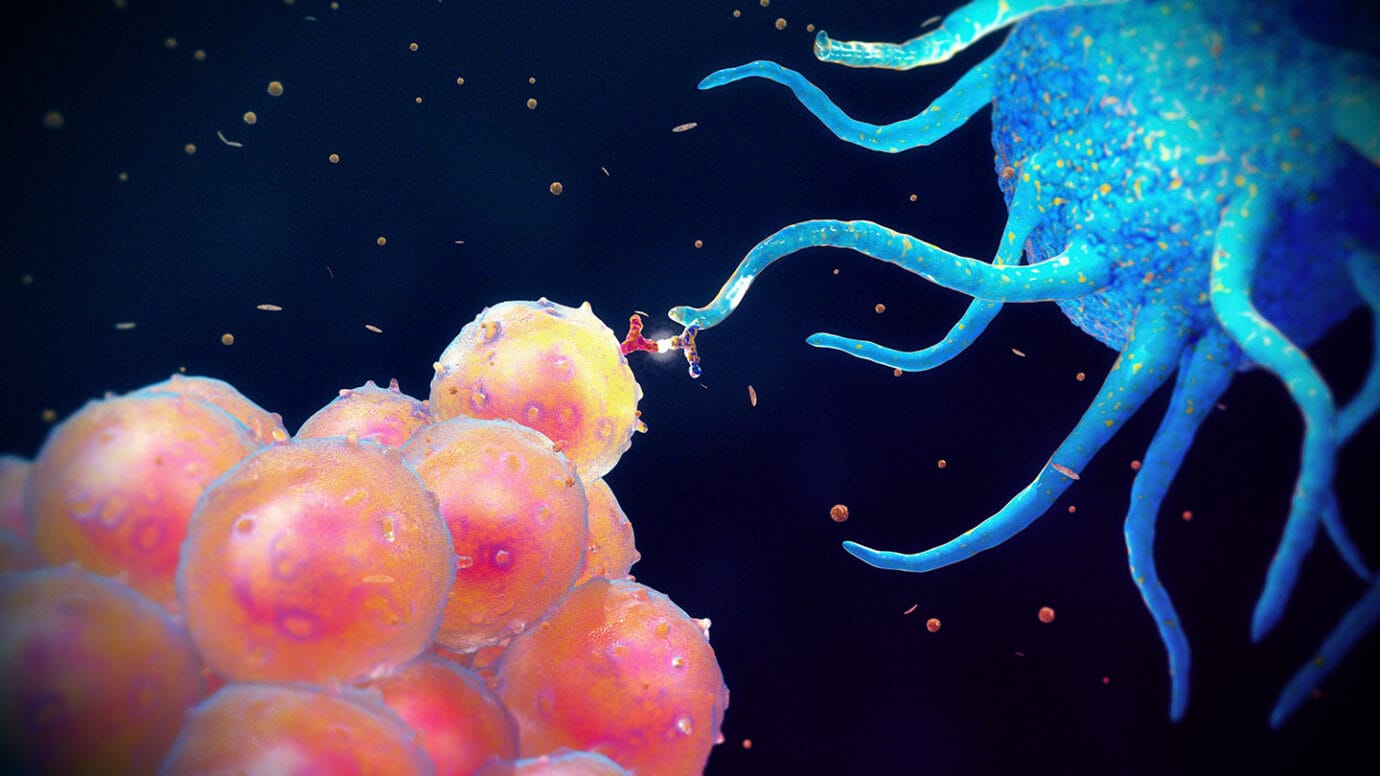
The Role of Dendritic Cells: Messengers of the Immune System
Among the most vital sentinels in immune detection are dendritic cells—often called the “bridge” between the innate and adaptive immune systems. These cells reside in the skin, lungs, and gut, constantly sampling their environment.
When dendritic cells encounter a pathogen, they engulf it, break it apart, and carry its pieces to nearby lymph nodes. There, they present the foreign antigens to T cells. This presentation—performed using MHC molecules—is what activates the adaptive immune response.
Dendritic cells don’t just deliver messages; they shape the entire immune reaction. They release specific cytokines that determine what kind of response is launched—whether it will be inflammatory, antibody-based, or geared toward cellular attack.
In this way, dendritic cells act as both detectives and dispatchers, alerting the immune army and telling it what kind of invader it’s dealing with.
The Gut and Mucosal Detection: A Delicate Balance
One of the most remarkable feats of immune detection happens in the gut. Every day, we ingest millions of microbes along with food. Many of them are harmless or even beneficial. So how does the immune system know when to attack and when to tolerate?
The gut-associated lymphoid tissue (GALT) is a complex network of immune cells located along the intestines. Specialized cells called M cells sample antigens from the gut lumen and pass them to underlying immune cells. If a dangerous pathogen is detected, an immune response is mounted. But if the substance is a harmless food protein or friendly bacterium, the system often induces oral tolerance—a state of non-responsiveness.
Failure in this delicate balance can result in inflammatory bowel disease, food allergies, or autoimmune reactions. It’s a stark reminder that detection isn’t just about identifying enemies. Sometimes, it’s about knowing when to stand down.
When Detection Fails: The Cost of Immunological Errors
The immune system’s power to detect invaders is immense—but not infallible. When it fails, the consequences can be catastrophic.
In immunodeficiency diseases, such as HIV/AIDS or congenital immune disorders, the body is unable to detect or respond to pathogens effectively. In cancer, some tumor cells manage to evade immune detection by downregulating MHC molecules or secreting immunosuppressive factors. And in autoimmune diseases, detection systems become tragically confused, attacking the very tissues they are meant to protect.
Even in everyday life, detection isn’t always perfect. Viruses like influenza or SARS-CoV-2 evolve rapidly, changing their surface proteins to escape recognition—a process known as antigenic drift. Our immune system must play catch-up, constantly updating its memory to face new threats.
The Future of Immune Detection: Vaccines, AI, and Synthetic Immunity
The more we learn about how the immune system detects invaders, the better we become at mimicking and enhancing it. Modern vaccines use pieces of pathogens—like mRNA or protein subunits—to train the immune system without causing illness. Immunotherapies are now being used to help the immune system detect and destroy cancer cells. Artificial intelligence is being used to predict new antigens and design vaccines in record time.
Scientists are even exploring synthetic immune systems—engineered cells that can detect diseases with unprecedented accuracy. As we harness the principles of immune detection, we are not just defending the body; we are learning to shape its future.
A Symphony of Surveillance
The detection of invaders by the immune system is not the job of a single cell or a single signal. It is a symphony—an orchestra of watchers, messengers, and killers, each playing a part in an ongoing performance that is as old as life itself.
Every time we survive a cold, every time a cut heals, every time a vaccine protects us, it is because the immune system recognized a threat and knew how to respond. Its ability to detect invaders is not just a biological function—it is a marvel of nature, a testament to the elegance and resilience of life.
And in every breath we take, it watches silently, patiently, ready to rise the moment danger comes near.