In the quiet chambers of the nose, a biological mystery has lingered for decades. How can a single sensory neuron—among billions—choose just one gene to express out of a thousand options, and stick to it for life? A new study published in Nature reveals an astonishing answer, one that not only reshapes our understanding of the sense of smell but also challenges the fundamental rules of molecular biology. The answer, it turns out, involves molecular structures that behave more like solids than liquids—a stunning deviation from what scientists thought possible in living cells.
The research, led by Dr. Stavros Lomvardas at Columbia University, explores how olfactory sensory neurons (OSNs) in the nose carry out what seems like an impossible task. These neurons are responsible for detecting odors, and each one expresses only a single olfactory receptor (OR) gene out of approximately 1,000. This precise expression is critical: it ensures that each neuron specializes in detecting a specific odor molecule, enabling the brain to interpret a vast array of smells with clarity and accuracy.
A Puzzle Hidden in Plain Sight
For decades, researchers have been fascinated by what’s known as “singular OR choice”—the molecular feat whereby one gene is selected and activated, while all others are permanently silenced. This selective activation is necessary for the brain to decode which neuron has been stimulated and thus what kind of odor has been detected. But the mechanism behind this singular choice has remained elusive, despite the fact that the genetic sequences involved were identified years ago.
Dr. Lomvardas, who has long been intrigued by this conundrum, reflects on what drew him to this mystery: “I am fascinated by the process of olfactory receptor choice and by the fact that these neurons deploy highly specific and extremely stable genomic interactions across chromosomes to achieve this goal.”
In other words, neurons don’t just pick a gene at random—they physically reorganize their genome, pulling together specific regulatory elements from across different chromosomes to build what scientists call “enhancer hubs.” The complexity of this process borders on the artistic: it’s as though the cell rearranges its DNA like a composer rearranging sheet music to produce a perfect symphony.
The Genome Folding Paradox
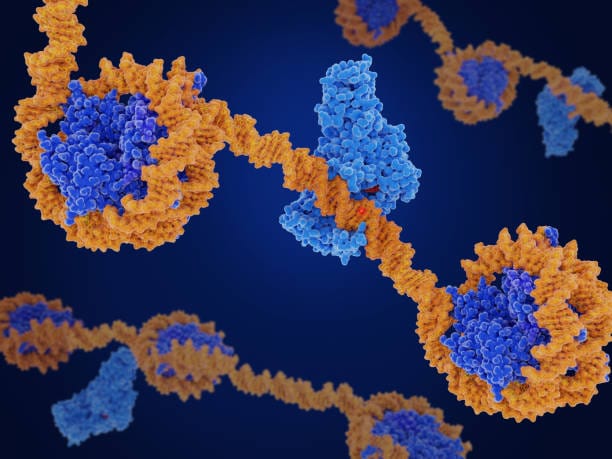
This cellular symphony gives rise to a deeper paradox in biology—something geneticists refer to as the “genome folding paradox.” Within the vast tangle of DNA inside every cell, some sequences called enhancers regulate which genes get turned on. These enhancers often lie far away—sometimes millions of base pairs—from the genes they control. And yet, remarkably, they find their target genes with laser-like precision while ignoring nearer, similar-looking sequences.
This flies in the face of basic molecular logic. In most cellular systems, proteins bind to the closest available site—proximity matters. But in olfactory neurons, distant regulatory elements appear to “know” where to go, skipping nearby genes to form long-range connections with a single target. This is not just biologically astonishing—it’s physically puzzling.
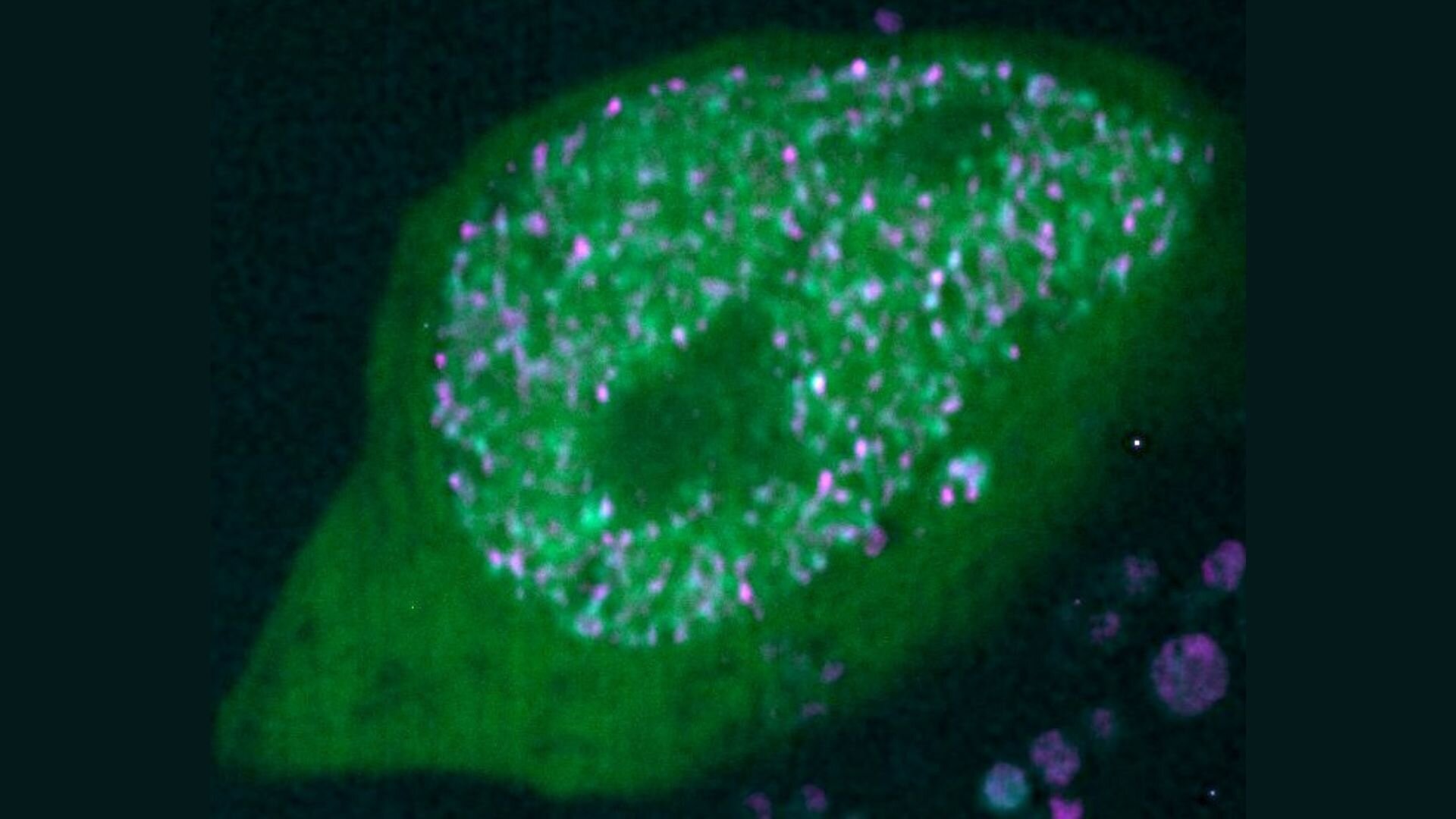
Previous research from Lomvardas’s lab showed that olfactory neurons cluster enhancer elements from multiple chromosomes into these three-dimensional “hubs” to activate just one olfactory receptor gene. But the biochemical glue that held these hubs together—what kept them stable and exclusive—was still a mystery.
Expecting Liquids, Finding Solids
To uncover this biochemical mystery, Lomvardas’s team focused on three key proteins: transcription factors LHX2 and EBF1, and an adaptor protein called LDB1. These molecules are known to regulate OR gene expression and were suspected to play a role in forming the mysterious enhancer hubs.
Drawing from modern cell biology, the team hypothesized that these proteins might undergo a process called liquid-liquid phase separation—a relatively new concept in biology that describes how certain proteins form droplet-like compartments in cells, much like oil separating from water. These compartments, called condensates, help organize cellular functions without relying on membranes.
“We initially thought we’d see liquid droplets,” said Lomvardas. “This was my preconceived notion going into the project.”
But the reality turned out to be far stranger. Using advanced protein purification techniques and live-cell imaging of cultured olfactory neurons, the researchers observed condensates forming—but they didn’t behave like liquids at all. Instead of merging fluidly or allowing molecules to drift in and out, these condensates held their shape. Even after ten minutes in a fluorescence recovery after photobleaching (FRAP) experiment—a test that typically shows rapid molecular movement—these structures showed no exchange of molecules. They were rigid. They were solid.
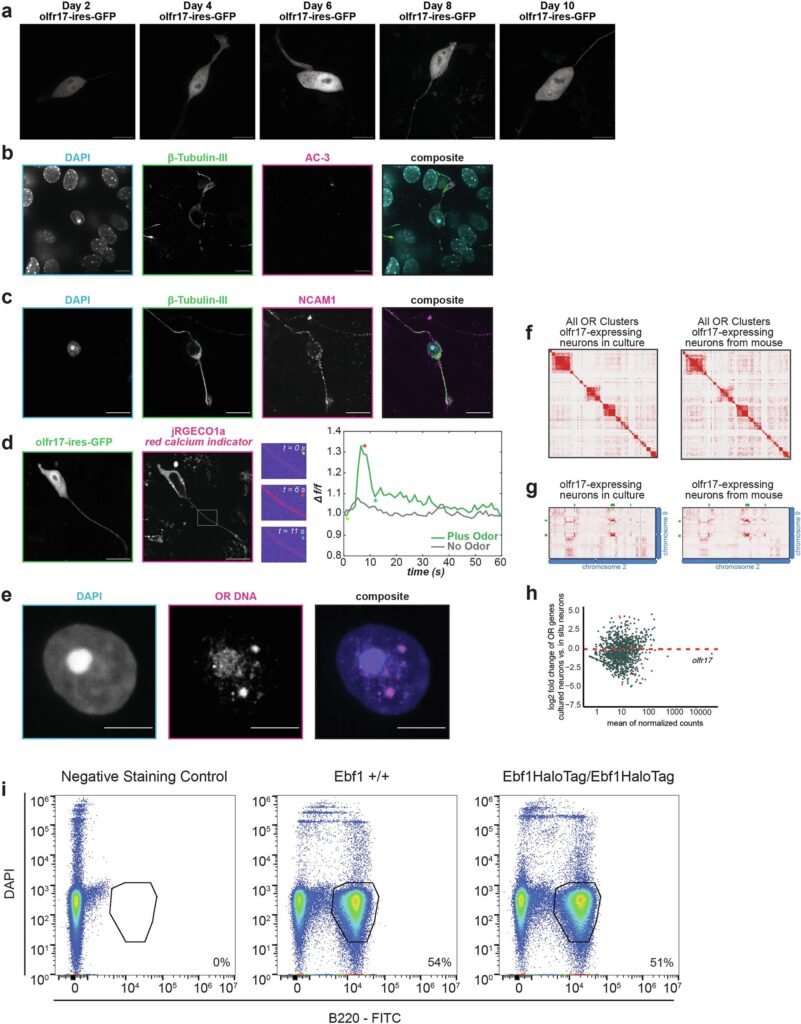
At first, the team thought it must be a mistake—a glitch in the experiment. But repetition brought the same results. These weren’t the liquid-like condensates seen in many other systems. These were solid-like molecular aggregates, forming a kind of biochemical crystal in the chaos of the cell.
DNA Patterns That Shape the Cell
What made these condensates form into solids rather than liquids? The answer, it turns out, was hidden in the DNA itself.
The researchers discovered that the enhancer sequences responsible for triggering solid condensate formation had a very specific pattern: a DNA sequence where the binding sites for LHX2 and EBF1 are separated by exactly one base pair. These “composite motifs,” as the team called them, appeared over and over in the enhancers of olfactory receptor genes—but not elsewhere.
“We knew from earlier work that these composite motifs were enriched in OR enhancers,” said Lomvardas. “But now we saw that they weren’t just involved in gene activation—they were defining the physical state of the protein-DNA complexes themselves.”
These motifs act like molecular Velcro. When the proteins bind to DNA sequences containing composite motifs, they create homophilic interactions—meaning they preferentially bind to other, identical protein-DNA complexes. This selective binding creates a kind of biochemical network, where only enhancers with the exact same motif can connect and form stable structures. Enhancers lacking the motif are excluded.
The result? A selective, rigid, and incredibly stable enhancer hub that activates one—and only one—olfactory receptor gene.
Greek Islands in the Genome
To see how this worked in real tissues, the team turned to 3D genome mapping techniques in mouse olfactory tissue. What they found was astonishing. Enhancers containing composite motifs formed ultra-long-range interactions, linking across chromosomes to form what the researchers called “Greek Islands.” These are spatial clusters of enhancer elements that reach out to specific OR genes, bypassing closer genes and ignoring enhancers that lack the necessary DNA motifs.
The metaphor is apt. Like islands in the Aegean Sea, these enhancer hubs are isolated, ancient, and intricately connected by invisible forces. And like mythic Greece, they guard their secrets closely. The solid-like condensates they form are so stable they resist dissolution by chemicals and remain intact for days. In a neuron that never divides and must remember its identity for a lifetime, this kind of permanence is a superpower.
A Broader Biological Principle?
While this study focuses on the olfactory system, its implications go far beyond the nose. The mechanism uncovered—where specific DNA sequences drive the formation of stable, solid-like condensates—may be a universal strategy used by cells that require permanent gene expression patterns. This is especially relevant for neurons, which are post-mitotic and must maintain their identity and function for decades.
“We are starting to realize that genomic interactions between DNA sequences that are megabases apart—or even between chromosomes—are not unique to olfactory neurons,” said Lomvardas. “Similar biochemical principles may be deployed in other cell types to orchestrate genome architecture and stabilize gene expression.”
Future research will explore these possibilities. Lomvardas’s team now plans to investigate the structural biology of these solid phase condensates—how they’re built, how they’re maintained, and where else they appear. There’s already speculation that similar structures may regulate genes in the immune system, the brain, and even cancer cells.
The Physics of Smell, Rewritten
In the end, this study redefines what we know about the physics of gene regulation. The idea that solid-like structures could exist within the fluid environment of the cell was once unthinkable. And yet, olfactory neurons are building these structures daily, using the language of DNA and the architecture of protein to create something more akin to crystal than soup.
In doing so, they not only ensure that your nose can detect a rose from a rotten egg, but also teach us that life’s most delicate processes are sometimes built on surprisingly solid ground.
And so, the next time you inhale the scent of coffee, or rain on pavement, or someone you love—know that a tiny structure inside your nose has chosen just one gene to make that moment possible. A single gene, held in place by a secret your body only now is beginning to understand.
Reference: Joan Pulupa et al, Solid phase transitions as a solution to the genome folding paradox, Nature (2025). DOI: 10.1038/s41586-025-09043-6.