Every second of every day, our bodies are locked in an invisible battle. Countless microscopic skirmishes unfold on the surfaces of our skin, in the fluids of our lungs, and in the corridors of our intestines. Bacteria—those ancient, single-celled survivors—are some of the main players in this biological war. Some are allies, quietly digesting food or outcompeting pathogens. Others are enemies, capable of triggering devastating infections. For most of human history, these infections were often deadly. But then, with the discovery of antibiotics in the 20th century, humanity gained a temporary upper hand. It was a triumph—a turning point in medicine that transformed once-fatal illnesses into treatable conditions.
However, that victory came with a caveat. Bacteria, with their immense evolutionary cunning and ability to adapt, began to push back. They developed resistance. One by one, antibiotics that once wiped out infections with ease began to lose their edge. Today, bacterial resistance is a growing crisis—threatening to send us spiraling back into an age where minor injuries and common infections become deadly again. But what exactly makes bacteria so capable of resisting the drugs meant to destroy them? What are the inner workings of these tiny organisms that allow them to adapt so rapidly and effectively?
To understand the full scope of this resistance, we must dive into the microscopic world of bacterial physiology, molecular warfare, and evolutionary strategy. Only then can we begin to grasp the gravity—and the hope—behind the story of bacterial resistance mechanisms.
The Genesis of Resistance: A Natural Evolutionary Tale
Bacterial resistance didn’t begin with humans. Long before Alexander Fleming discovered penicillin in 1928, long before human medicine emerged, bacteria were already producing and resisting antibiotics. In fact, antibiotics are natural compounds, originally created by microorganisms—like fungi or other bacteria—to eliminate competitors in the soil or water.
In this primordial arms race, bacteria developed resistance mechanisms not out of malice but survival. It was evolution in its purest form: variation, selection, inheritance. Any bacterium that stumbled upon a genetic mutation or acquired a new gene enabling it to withstand an antibiotic-like substance had a better chance of survival. Over time, these genes became refined, diversified, and spread among microbial populations.
So when humans began using antibiotics—first penicillin, then streptomycin, tetracycline, and many others—we unintentionally tapped into an ancient, ongoing battle. The resistance genes were already out there, dormant or rare. But the sudden explosion of antibiotic use created a powerful selective pressure. The bacteria with resistance genes flourished, while their susceptible counterparts were eliminated. Thus began a new chapter in the microbial arms race, one with profound consequences for human health.
How Bacteria Resist: The Molecular Defense Playbook
Bacteria do not think. They have no consciousness, no strategy meetings, no blueprints. Yet they exhibit some of the most elegant and efficient defense systems known in biology. Their resistance mechanisms fall into a few broad categories—each one a marvel of molecular ingenuity.
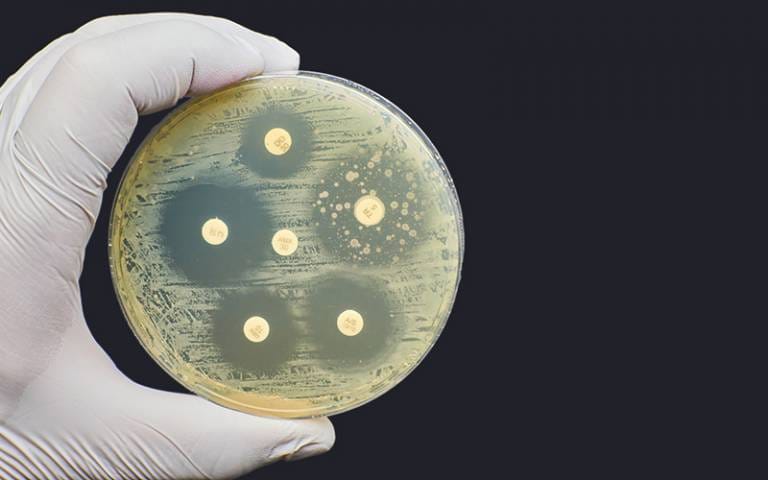
One of the most common methods bacteria use to evade antibiotics is to alter the drug’s target. Most antibiotics work by binding to specific proteins or structures within the bacterial cell—such as the ribosome, cell wall, or DNA enzymes. A small genetic mutation that subtly changes the shape or configuration of these targets can render the drug ineffective. It’s like changing the lock so the old key no longer fits.
Another strategy involves actively ejecting the antibiotic from the bacterial cell. Specialized protein pumps, embedded in the bacterial membrane, act like miniature evacuation systems—identifying harmful substances and expelling them before they can do any damage. These efflux pumps are often non-specific, meaning they can protect against multiple types of antibiotics at once, making them especially dangerous.
Some bacteria go further and produce enzymes that directly destroy the antibiotic. Beta-lactamases, for example, are enzymes that cleave the molecular structure of beta-lactam antibiotics like penicillin, making them useless. These enzymes are not rare—bacteria can produce them in large quantities and even share the genetic instructions for making them with their neighbors.
A more elusive form of resistance is the ability to hide or enter a dormant state. Certain bacterial species can form biofilms—dense, slimy communities where cells are protected by an extracellular matrix. Inside a biofilm, antibiotics have trouble penetrating, and bacterial metabolism slows, reducing the drug’s effectiveness. Alternatively, bacteria may become “persisters,” entering a temporary, non-replicating state in which antibiotics that target growing cells become ineffective. These sleeper agents can later reawaken and re-infect the host.
Each of these strategies offers bacteria a lifeline. Combined, they create a formidable, adaptive defense system—one that continues to evolve and confound modern medicine.
Sharing the Tools of Survival: Horizontal Gene Transfer
Perhaps the most astonishing ability of bacteria is their capacity to share genetic material—not just with their offspring, but with other bacteria, even those of different species. This process, known as horizontal gene transfer, is one of the key drivers of antibiotic resistance.
There are three main modes of horizontal gene transfer. Transformation involves bacteria picking up free-floating DNA from their environment—often released by dead cells. Transduction occurs when viruses that infect bacteria (bacteriophages) inadvertently transfer DNA between cells. But the most impactful method is conjugation, a process in which bacteria form a physical connection—like a molecular handshake—through which they transfer plasmids, circular DNA molecules carrying resistance genes.
This microbial generosity allows resistance traits to spread rapidly across populations and ecosystems. A harmless soil bacterium might pass a resistance gene to a gut microbe. A harmless gut microbe might then share that gene with a pathogenic strain. In hospitals, where antibiotics are used heavily, bacteria are under intense selection pressure, and horizontal gene transfer turns them into hubs of evolutionary innovation.
It’s a microbial version of social networking—one that’s alarmingly efficient and extraordinarily fast. Where human evolution takes millennia, bacterial populations can evolve significant resistance in a matter of weeks or months. And in doing so, they become increasingly difficult to treat.
Clinical Crisis: When the Drugs Stop Working
The impact of bacterial resistance is not an abstract future threat—it’s a real, unfolding crisis. Each year, hundreds of thousands of people around the world die from infections that were once treatable. Simple procedures—appendectomies, cesarean sections, even dental surgeries—carry renewed risks when infections become untreatable.
Diseases once considered under control are making a comeback. Tuberculosis, for instance, has reemerged with multidrug-resistant (MDR) and extensively drug-resistant (XDR) forms, particularly in parts of Asia and Africa. Gonorrhea, once curable with a single injection, has developed strains that resist nearly every antibiotic in our arsenal. Hospital-acquired infections caused by bacteria like Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii are increasingly difficult to treat, particularly in intensive care units.
The World Health Organization and Centers for Disease Control and Prevention have repeatedly declared antibiotic resistance a top global health threat. Without action, experts predict that by 2050, resistant infections could cause more deaths annually than cancer. Economically, the cost is staggering—billions of dollars in extended hospital stays, additional treatments, and lost productivity.
But the most devastating impact is personal. Families torn apart by preventable deaths. Patients battling infections for months. Healthcare providers forced to watch helplessly as once-routine cases spiral into medical tragedies. The human cost is immeasurable.
Agriculture and the Hidden Spread of Resistance
While hospitals are epicenters of resistant infections, the problem begins far beyond their walls. One of the most overlooked drivers of resistance is the widespread use of antibiotics in agriculture. In many countries, antibiotics are routinely given to livestock not just to treat disease, but to promote faster growth and prevent potential illness in crowded conditions.
This overuse creates ideal conditions for resistant bacteria to emerge and flourish within animal populations. These bacteria can then spread to humans through food, water, and direct contact. Resistant genes developed in the gut of a pig or chicken can, through horizontal gene transfer, find their way into human pathogens.
Antibiotic residues from agricultural runoff also contaminate soil and waterways, exerting selective pressure on environmental bacteria. This, in turn, creates vast microbial reservoirs of resistance genes that can later be recruited by human pathogens.
In effect, our food production systems have become breeding grounds for superbugs. The cheap meat we buy at the store may carry with it invisible risks—resistant bacteria capable of undermining the very medicines we rely on to stay alive.
Searching for Solutions: Science Fights Back
Despite the grim realities, the story of bacterial resistance is not a hopeless one. Scientists, doctors, public health officials, and policymakers are actively working to counter the threat. But it requires a multi-pronged strategy that goes far beyond developing new antibiotics.
Research into alternative treatments is intensifying. Bacteriophage therapy, which uses viruses that specifically infect and kill bacteria, is one promising avenue. Because phages are highly specific to their bacterial hosts, they can target pathogens without harming beneficial microbes—an advantage over broad-spectrum antibiotics.
Other scientists are exploring antimicrobial peptides, small protein molecules that disrupt bacterial membranes, as potential drugs. There’s also growing interest in anti-virulence therapies—drugs that disarm bacteria without killing them, reducing the selective pressure for resistance. These treatments may inhibit toxin production, prevent biofilm formation, or block the bacteria’s ability to communicate through quorum sensing.
Meanwhile, advances in genomics and machine learning are helping researchers identify resistance genes faster, develop diagnostic tools to guide precise antibiotic use, and even design synthetic molecules that evade known resistance mechanisms.
Yet scientific innovation alone is not enough. Global stewardship efforts—like regulating antibiotic use in agriculture, improving sanitation and vaccination, and ensuring proper antibiotic prescriptions—are essential to buying time and preserving the drugs we already have.
Personal Responsibility and Global Consequences
Though bacterial resistance is a global crisis, individual choices matter. When patients demand antibiotics for viral infections like colds or the flu, when prescriptions are not taken as directed, or when leftover antibiotics are saved for later use, the result is not just a missed treatment—it’s an opportunity for bacteria to evolve.
Every unnecessary dose contributes to the broader problem. Educating the public about responsible antibiotic use is one of the simplest yet most impactful tools we have. Likewise, healthcare providers must be supported with diagnostics, training, and time—so they can resist the pressure to prescribe antibiotics when they’re not needed.
At the same time, global inequities must be addressed. In some regions, antibiotics are overprescribed; in others, they are inaccessible or counterfeit. Solving bacterial resistance means ensuring that everyone, everywhere, has access to the right drug at the right time—and that new drugs, once developed, are not squandered.
The Hope Within the Microbe
Bacteria are not our enemies. They were here billions of years before us and will likely persist long after we’re gone. The vast majority are harmless or even essential to life. Our own bodies contain more bacterial cells than human cells. They help us digest food, produce vitamins, and train our immune systems. The war we fight against bacterial resistance is not a war against bacteria themselves—it’s a struggle to preserve balance, to outwit the forces of evolution that, when unchecked, can turn allies into adversaries.
In understanding bacterial resistance mechanisms, we confront some of the most profound questions in biology. How do living systems adapt to threats? How do simple organisms develop complex strategies without intention or design? And how can we, as a species, use our intelligence not just to dominate nature, but to live in harmony with it?
The answers lie not just in the laboratory, but in our policies, our ethics, and our collective will to act. Bacterial resistance is not a single problem with a single solution—it is a mirror reflecting our relationship with the natural world. Whether we learn to navigate that relationship wisely will define the health of future generations.