Every square inch of the human body, every breath of air, and every drop of water teems with life too small to see. Bacteria—those minuscule, single-celled organisms—have existed for over three billion years. They inhabit nearly every corner of the Earth, from boiling hot springs to frozen tundras, from our own intestines to the vastness of the ocean floor. Though invisible to the naked eye, these ancient organisms are the engines of ecosystems, the builders and destroyers of health, and the subjects of unending scientific fascination.
In microbiology, the laboratory becomes the gateway to this unseen world. Culturing and identifying bacteria is a blend of art and science, intuition and precision. It requires careful technique, patience, and an inquisitive mind ready to peer into the mysterious dance of microbial life. Whether you’re diagnosing disease, testing environmental samples, or uncovering new species, the process of culturing and identifying bacteria in the lab is a foundational skill—one that bridges observation with molecular discovery.
The Journey Begins: Collecting and Preparing Samples
The very first step in working with bacteria is knowing where to look and how to collect them. Sampling is not merely a technical task—it’s an act of curiosity. You might swab a wound, test contaminated water, or press a fingerprint onto a petri dish. Each sample holds the potential to reveal complex bacterial ecosystems or single deadly pathogens.
When collecting samples for culture, contamination is the enemy. Sterile techniques—using gloves, flame-sterilized tools, and sealed containers—help ensure that only the bacteria of interest are cultured. In clinical settings, the stakes are high: a sample taken improperly can mislead diagnosis or obscure the presence of dangerous microbes. Thus, care, clarity, and context are everything.
Once the sample is secured, it must be properly transported and processed. Time is critical. Some bacteria are fragile and die quickly outside the body. Others are hardy and flourish even under extreme conditions. Refrigeration may slow down bacterial metabolism and preserve the integrity of the sample, while nutrient buffers can stabilize its environment until culture.
Creating the Right Environment: Choosing Culture Media
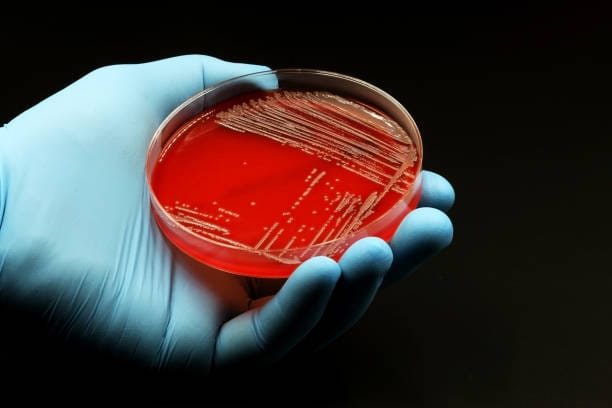
Bacteria, like people, thrive under the right conditions. In the lab, those conditions are recreated using culture media—nutrient-rich substances designed to support microbial growth. Choosing the right medium is both a science and a strategy. Are you trying to grow everything in the sample? Then you might use a general-purpose medium like nutrient agar or tryptic soy agar. Are you trying to isolate a specific species, like Salmonella or Staphylococcus? Then selective and differential media come into play.
Agar, derived from red algae, is the most common solidifying agent in bacterial culture. It melts when heated and solidifies as it cools, forming a gel that supports colony formation. Into this gel, scientists mix nutrients, salts, and sometimes dyes or antibiotics that help target specific bacteria.
MacConkey agar, for instance, allows Gram-negative bacteria to grow while inhibiting Gram-positive species. It also differentiates between lactose fermenters and non-fermenters, with color changes revealing the metabolic nature of the bacteria. Blood agar, on the other hand, supports a wide range of fastidious organisms and can indicate hemolytic activity, a sign of potential pathogenicity.
Each plate poured, each tube inoculated, is a silent invitation—offering bacteria a chance to reveal themselves.
Incubation: Letting Life Unfold
Once inoculated, culture plates or tubes are placed in incubators—controlled environments that mimic the warmth and conditions many bacteria prefer. Human pathogens, for instance, often grow best at 35–37°C, the internal temperature of the body. Others may require cooler or warmer settings, or specific atmospheric gases like CO₂ or oxygen-free environments for anaerobes.
The act of incubation is deceptively simple. Dishes are placed in racks, doors are closed, and time begins. But beneath the surface, something astonishing is happening. Bacteria, often just a few cells at the start, begin to divide—doubling every 20 minutes in some cases. Within hours or days, visible colonies emerge—tiny, shimmering discs that paint the once-sterile agar with signs of life.
To watch bacteria grow is to witness a miracle of biology. Each colony arises from a single progenitor cell, duplicating its DNA, building cell walls, producing enzymes, and interacting with the medium around it. The shapes, colors, edges, and textures of these colonies begin to tell their own stories.
Morphology: Reading the Patterns of Growth
Colony morphology is an art as much as a diagnostic tool. Trained microbiologists can often identify a bacterial genus by simply looking at the shape, size, and hue of colonies. Are they smooth or rough? Round or spreading? Creamy white or blood-red? Do they emit odors or change the color of the agar beneath them?
Staphylococcus aureus typically forms golden-yellow colonies, while Escherichia coli produces moist, grayish growths. Pseudomonas aeruginosa can emit a fruity or grape-like odor and display a metallic sheen. Bacillus species might grow in large, flat, irregular colonies, hinting at their spore-forming nature.
While morphology is not definitive, it provides valuable first impressions. It helps narrow down possibilities, especially when combined with the type of media used and the sample’s clinical or environmental background.
Microscopy adds another layer of detail. A simple Gram stain, invented by Hans Christian Gram in 1884, remains one of the most powerful techniques in bacteriology. It divides bacteria into two groups: Gram-positive (which retain a violet stain) and Gram-negative (which appear pink or red). This distinction reveals key structural differences in bacterial cell walls and guides both diagnosis and treatment.
Beyond Gram staining, special stains like acid-fast staining (for Mycobacterium), endospore stains, and capsule stains uncover more secrets about bacterial identity and behavior.
Biochemical Testing: Probing the Inner Workings
While appearance can suggest, biochemical tests confirm. These tests examine how bacteria interact with nutrients, enzymes, and chemicals. Each species has a unique metabolic fingerprint—a set of capabilities and limitations that help distinguish it from others.
Some tests are simple. Does the bacterium ferment glucose? Produce gas? Break down urea? Others are more complex, involving a series of reactions in tubes, wells, or dipsticks that change color in response to bacterial enzymes.
The catalase test, for example, checks whether bacteria produce the enzyme catalase, which breaks down hydrogen peroxide into water and oxygen. A positive result is revealed by bubbling—a mini-explosion of gas indicating microbial metabolism. The oxidase test identifies bacteria that possess cytochrome c oxidase, a component of the electron transport chain.
Sugar fermentation tests, indole production, citrate utilization, hydrogen sulfide formation—each of these biochemical puzzles helps narrow down the identity of a bacterial species. Often, laboratories use commercially available panels, like the API or Enterotube systems, that combine dozens of tests in one compact format. These panels generate patterns of positive and negative results that can be matched to identification charts or software databases.
Molecular Techniques: Reading the Genetic Code
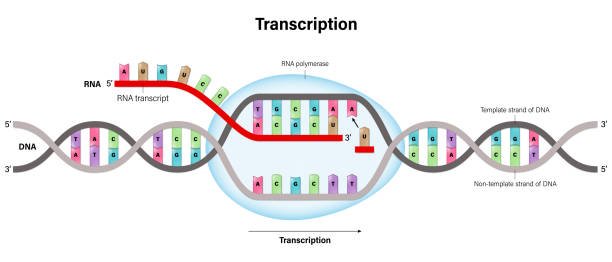
In the modern lab, identification doesn’t stop with stains and chemical reactions. DNA-based methods have revolutionized bacteriology, offering accuracy, speed, and insights that were once impossible.
Polymerase chain reaction (PCR) allows scientists to amplify and detect specific genetic sequences in bacterial DNA. It’s like having a molecular search engine that can find a needle in a genomic haystack. Real-time PCR even quantifies bacterial load, helping measure the severity of infections or track contamination.
16S ribosomal RNA gene sequencing is another powerful tool. The 16S rRNA gene, present in all bacteria, contains both conserved and variable regions. By comparing the sequence of this gene in an unknown bacterium to databases of known species, scientists can achieve precise identification—even discovering new, unculturable microbes in the process.
More recently, whole-genome sequencing (WGS) has become feasible in routine labs. This technology reads the entire genetic code of bacteria, allowing not just identification, but prediction of antibiotic resistance, virulence factors, and evolutionary relationships. With tools like bioinformatics, microbiologists can now ask deeper questions: How did this strain evolve? Where did it come from? What can it do?
Antibiotic Sensitivity Testing: Choosing the Right Weapon
Identifying a bacterium is only half the battle—especially in medicine. Knowing how to treat it is equally critical. Antibiotic sensitivity testing (AST) helps determine which drugs can inhibit or kill a specific bacterial strain.
One of the most widely used methods is the Kirby-Bauer disk diffusion test. A lawn of bacteria is spread across an agar plate, and paper disks impregnated with antibiotics are placed on the surface. As the bacteria grow, clear zones—called zones of inhibition—form around effective antibiotics. Measuring these zones tells scientists which drugs are likely to work.
Minimum inhibitory concentration (MIC) testing, using broth dilutions or automated systems, provides a more quantitative measure—telling precisely how much antibiotic is needed to halt bacterial growth.
In an age of rising antibiotic resistance, AST is more important than ever. Bacteria like methicillin-resistant Staphylococcus aureus (MRSA) or carbapenem-resistant Enterobacteriaceae (CRE) pose serious threats. Culturing and identifying these strains, alongside sensitivity testing, informs doctors, protects patients, and guides public health responses.
Identification in the Field: Clinical, Environmental, and Industrial Microbiology
While lab procedures are standardized, the context of bacterial identification varies widely. In clinical microbiology, time is of the essence. A bloodstream infection, urinary tract infection, or pneumonia must be identified quickly so treatment can begin. Hospitals use rapid diagnostic platforms that combine culture, biochemistry, and molecular methods.
In environmental microbiology, scientists may culture bacteria from soil, water, or air to monitor pollution, assess ecosystem health, or detect pathogens in agriculture and food. Industrial microbiology, on the other hand, might identify bacterial contaminants in pharmaceutical products or use engineered strains for fermentation and biotechnology.
No matter the setting, the goals are the same: to understand, to detect, and to harness or fight bacteria for the benefit of society.
The Art of Interpretation
Culturing and identifying bacteria is not a mechanical checklist—it’s a dialogue with the invisible. Each sample brings its own mysteries. Is a skin swab showing normal flora or an opportunistic pathogen? Is a water sample revealing environmental bacteria or fecal contamination? Are two similar colonies from different species, or are they mutants of the same strain?
Microbiologists must be detectives—observing, hypothesizing, testing, and questioning. They must guard against contamination, interpret results in context, and remain aware of limitations. A false positive can lead to unnecessary treatments; a false negative can miss a deadly pathogen. Experience, training, and critical thinking are vital.
In teaching labs, students often make mistakes: inoculating the wrong media, over-incubating a sample, misreading a test. But these are the moments where learning happens. To culture bacteria is to engage with life at its most elemental—and to realize how delicate and powerful that life can be.
Ethical Dimensions and Emerging Technologies
With greater power comes greater responsibility. As laboratory techniques grow more advanced, ethical questions emerge. Should genetically modified bacteria be released into the environment to clean oil spills or fix nitrogen in crops? How do we ensure that lab workers are protected from dangerous strains? What safeguards exist to prevent bioterrorism or misuse?
Meanwhile, new technologies continue to push boundaries. Lab-on-a-chip devices now allow culture and identification in minutes using microfluidics. AI-powered systems can analyze colony morphology or sequencing data faster than any human. Portable PCR machines are used in disaster zones, space stations, and developing regions.
But at the heart of it all remains a simple, enduring practice: taking a sample, placing it on a dish, watching it grow.
Conclusion: A Window Into the Microbial Cosmos
The practice of culturing and identifying bacteria is more than a technical skill. It is a voyage—one that takes us from the macroscopic world of symptoms, soil, and surfaces into the microscopic universe of DNA, enzymes, and cellular machinery. It is where science becomes tangible, where questions become visible, and where the abstract becomes personal.
Whether diagnosing a sick child, tracing a foodborne outbreak, or exploring the origins of life, microbiologists act as translators between worlds. In the quiet hum of incubators and the soft glow of stained slides, they listen to what bacteria have to say. And in their careful work, they remind us that even the smallest forms of life can teach us the biggest lessons—about survival, complexity, connection, and the ceaseless creativity of nature.