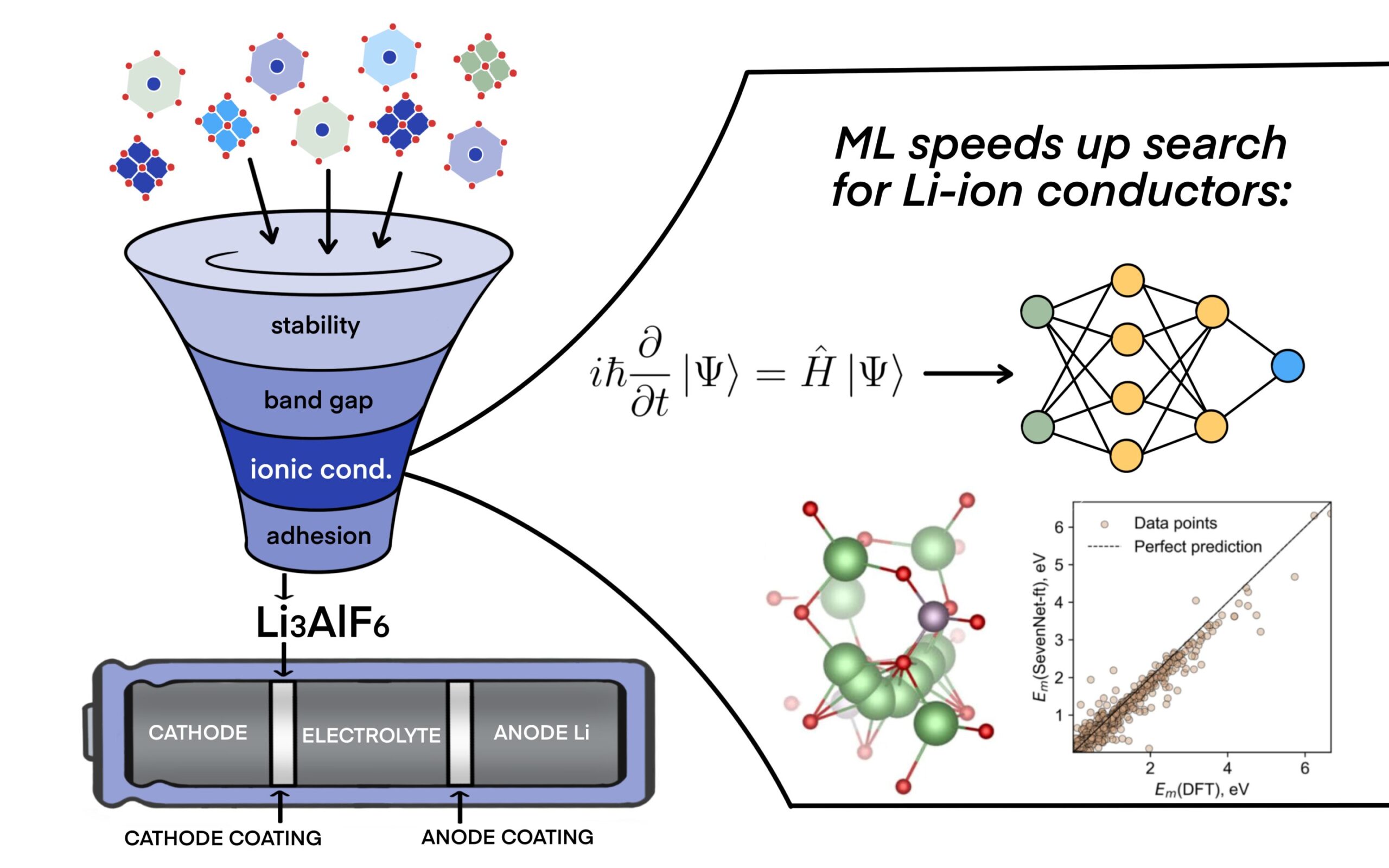
The future of energy may hinge on something as small as an ion—and as complex as a neural network.
In a remarkable fusion of materials science and artificial intelligence, researchers from Skoltech and the AI Research Institute (AIRI) have unveiled a machine learning framework that dramatically accelerates the search for next-generation solid-state lithium-ion batteries. The study, recently published in npj Computational Materials, isn’t just a technical upgrade—it may be a key stepping stone toward safer, longer-lasting electric vehicles and electronics.
At the heart of their innovation lies a novel application of graph neural networks (GNNs), a type of machine learning model that interprets the atomic structure of materials in a fundamentally smarter way. In doing so, the researchers demonstrated how AI can streamline the grueling process of identifying ideal materials for the elusive solid electrolyte and its protective coatings—components that could make or break the future of solid-state battery technology.
A Revolution Waiting to Ignite
The stakes are high. Solid-state batteries, if successfully commercialized, could solve several of the most nagging issues in lithium-ion technology. Unlike today’s batteries, which rely on flammable liquid electrolytes that ferry lithium ions between the electrodes, solid-state batteries use solid materials—often ceramics or glassy compounds—to perform the same job. This shift comes with several theoretical advantages: they’re non-flammable, have a longer lifespan, and can potentially store more energy in a smaller space.
In practical terms, this could translate to electric vehicles (EVs) with dramatically extended range—possibly up to 50% more than what current batteries allow—and electronics that hold a charge for days, not hours. And all this could come without the fire risks that have plagued everything from smartphones to airplanes.
But there’s a catch.
“We still don’t have a single solid electrolyte that ticks all the boxes,” says Artem Dembitskiy, the study’s lead author and a PhD student at Skoltech’s Materials Science and Engineering program. “The materials we’ve discovered so far either have poor ionic conductivity, degrade when in contact with electrodes, or are too unstable to manufacture economically.”
The roadblock lies in the materials. To develop a commercially viable solid-state battery, scientists need to find a solid electrolyte that not only conducts lithium ions efficiently but also remains chemically and mechanically stable in the harsh electrochemical environment inside a battery. And that’s not easy.
The Challenge of Materials Discovery
To outsiders, finding a new battery material may sound like tweaking a few ingredients and running some lab tests. In reality, it’s more like searching for a needle in an infinitely complex haystack—one composed of millions of hypothetical chemical compounds, each with unpredictable properties and unstable behaviors under different conditions.
Traditionally, scientists have used ab initio quantum chemistry calculations—essentially solving the Schrödinger equation for every potential material configuration—to predict properties like ionic conductivity, thermodynamic stability, and electronic behavior. These methods are precise but painfully slow. Evaluating just a few hundred candidate materials can take months, even on powerful supercomputers.
Enter machine learning.
Rather than solving physics equations from scratch for each material, AI models like graph neural networks learn to “predict” those properties by recognizing patterns in known data. Once trained, they can evaluate thousands—or even millions—of candidate materials in a fraction of the time it would take traditional simulations. It’s like going from handcrafting every piece of a puzzle to using an algorithm that knows where each piece probably belongs.
Graph Neural Networks: A New Kind of Microscope
So what exactly makes GNNs so powerful in this context?
Unlike other neural networks that operate on image pixels or time series data, graph neural networks work directly with the fundamental structure of matter: atoms and their bonds. Each atom is represented as a node, and the chemical bonds between them as edges in a graph. The GNN “learns” how atoms interact by training on datasets of known materials, developing an intuitive understanding of how atomic configurations affect properties like conductivity or chemical stability.
In the Skoltech–AIRI study, the researchers trained their GNNs to predict ionic conductivity—a key property that determines how easily lithium ions can migrate through a material. High ionic conductivity is critical for solid electrolytes and equally important for the protective coatings that must endure extreme chemical environments inside the battery.
The results were striking. The model could predict ionic conductivity orders of magnitude faster than traditional quantum chemistry simulations, and with comparable accuracy. And not just for bulk materials: the team extended the method to search for interface coatings as well.
Solving the Interface Problem
Even if scientists discover the “perfect” solid electrolyte, it may still fail in a real battery. The reason? Interfaces.
The solid electrolyte must sit snugly between the battery’s two electrodes: a metallic lithium anode on one side and a high-voltage cathode on the other. Both are chemically aggressive. Metallic lithium is a powerful reducing agent—it likes to give away electrons and break down whatever touches it. The cathode, on the other hand, is a strong oxidizer, eager to snatch electrons.
This chemical squeeze turns solid electrolytes into molecular battlegrounds. Without protection, the electrolyte can degrade at either interface, forming resistive layers, losing mechanical integrity, or even short-circuiting the battery.
“You can avoid this by introducing two protective coatings,” explains co-author Dmitry Aksyonov, an assistant professor at Skoltech Energy. “One stable against lithium at the anode, and another stable against the cathode. The coatings act like diplomatic buffers, allowing the materials to coexist without reacting violently.”
But finding suitable coatings is yet another complex problem—one with its own laundry list of required properties: thermodynamic stability, electronic insulation, electrochemical compatibility, high lithium-ion mobility, and more. That’s where the GNN model stepped in once again.
A New Pipeline for Discovery
To test their model, the researchers focused on a particularly promising solid electrolyte: Li₁₀GeP₂S₁₂ (LGPS). Known for its exceptional ionic conductivity—almost on par with liquid electrolytes—LGPS has long been a darling of solid-state battery research. But it’s also notoriously unstable at electrode interfaces.
Using their GNN-powered pipeline, the team screened thousands of candidate materials as potential protective coatings for LGPS. The model rapidly filtered out those with poor ionic conductivity or unfavorable electrochemical windows, narrowing the list to just a few promising candidates.
Among the top hits: Li₃AlF₆ and Li₂ZnCl₄—both compounds that had not been previously highlighted for this application. These materials showed the right combination of stability, conductivity, and manufacturability to warrant further investigation in experimental settings.
Accelerating the Battery Race
The implications of this research go well beyond a few candidate materials. What Skoltech and AIRI have demonstrated is a scalable, generalizable framework for accelerating battery materials discovery. Instead of brute-forcing millions of quantum calculations, researchers can now let AI do the heavy lifting—reserving costly simulations and experiments for only the most promising leads.
This matters because the competition to commercialize solid-state batteries is already heating up. Automotive giants like Toyota, Volkswagen, and BMW have all invested heavily in solid-state research, hoping to leapfrog current limitations and secure the next generation of EV technology. Startups like QuantumScape and Solid Power are also racing to market with their own proprietary designs.
But the materials bottleneck remains.
By merging deep learning with computational chemistry, the Skoltech–AIRI team offers a new path forward—one where progress is limited not by CPU cycles, but by creativity and vision.
Beyond Batteries: A New Era for Materials Science
There’s a deeper story here, too—one that goes beyond batteries.
Materials science has long been a slow and meticulous field, governed by trial and error, serendipity, and slow simulations. But with AI entering the lab, the game is changing. Algorithms can now explore chemical spaces so vast they defy human intuition, guiding scientists toward novel materials for everything from semiconductors and superconductors to catalysts and carbon capture.
We are witnessing the dawn of an era where neural networks become creative partners in discovery, not just tools for automation. As this collaboration deepens, we may find that the smartest materials of tomorrow aren’t born solely from human hands—but also from artificial minds trained to understand the fundamental language of matter.
And if that happens, the battery breakthroughs we dream of today may just be the beginning.
Reference: Artem D. Dembitskiy et al, Benchmarking machine learning models for predicting lithium ion migration, npj Computational Materials (2025). DOI: 10.1038/s41524-025-01571-z