From the smartphone in your pocket to the electric car silently gliding down the highway, lithium-ion batteries (LIBs) are the beating heart of modern mobility and communication. These compact energy storage devices power everything from laptops to drones and renewable energy grids. But inside their sleek casings lies a dangerous secret: the ever-present risk of thermal runaway—a chain reaction of heat and gas buildup that can turn your battery into a mini firebomb.
It’s not science fiction. Thermal runaway is the culprit behind headline-making fires in hoverboards, laptops, and even commercial aircraft. As our world becomes increasingly electrified, the demand for safer, smarter batteries has never been more urgent. Now, researchers at IMDEA Materials Institute in Spain are stepping up with an ingenious solution—one that doesn’t just resist fire, but thinks ahead to prevent it.
A Smarter Solution: Turning Chemistry into a Guardian
Led by Dr. Arnab Ghosh, a materials scientist specializing in battery safety, a team of researchers at IMDEA Materials has developed a breakthrough thermoresponsive electrolyte—a substance that responds intelligently to heat inside the battery, shutting down potential fires before they start.
Their work, published in Advanced Functional Materials, is the flagship result of the SMARTBATT project, funded under the Marie Skłodowska-Curie Actions program. The timing couldn’t be better: the European Commission’s Battery 2030+ initiative is calling for precisely this kind of innovation—batteries that are not only powerful and long-lasting, but fundamentally safe.
At the center of the discovery is a radical rethinking of battery design. Traditional LIBs rely on a passive safety mechanism: a separator—typically a polypropylene/polyethylene (PP/PE/PP) trilayer—that melts at around 160°C, cutting off ion flow to prevent the battery from overheating further. But in real-world conditions, LIBs can reach temperatures well above 160°C before this safety feature kicks in. By that time, it’s often too late.
This is where thermoresponsive electrolytes enter the scene—not as a secondary safety layer, but as an intelligent core component that reacts before things spiral out of control.
The Chemistry of Control: How the New Electrolyte Works
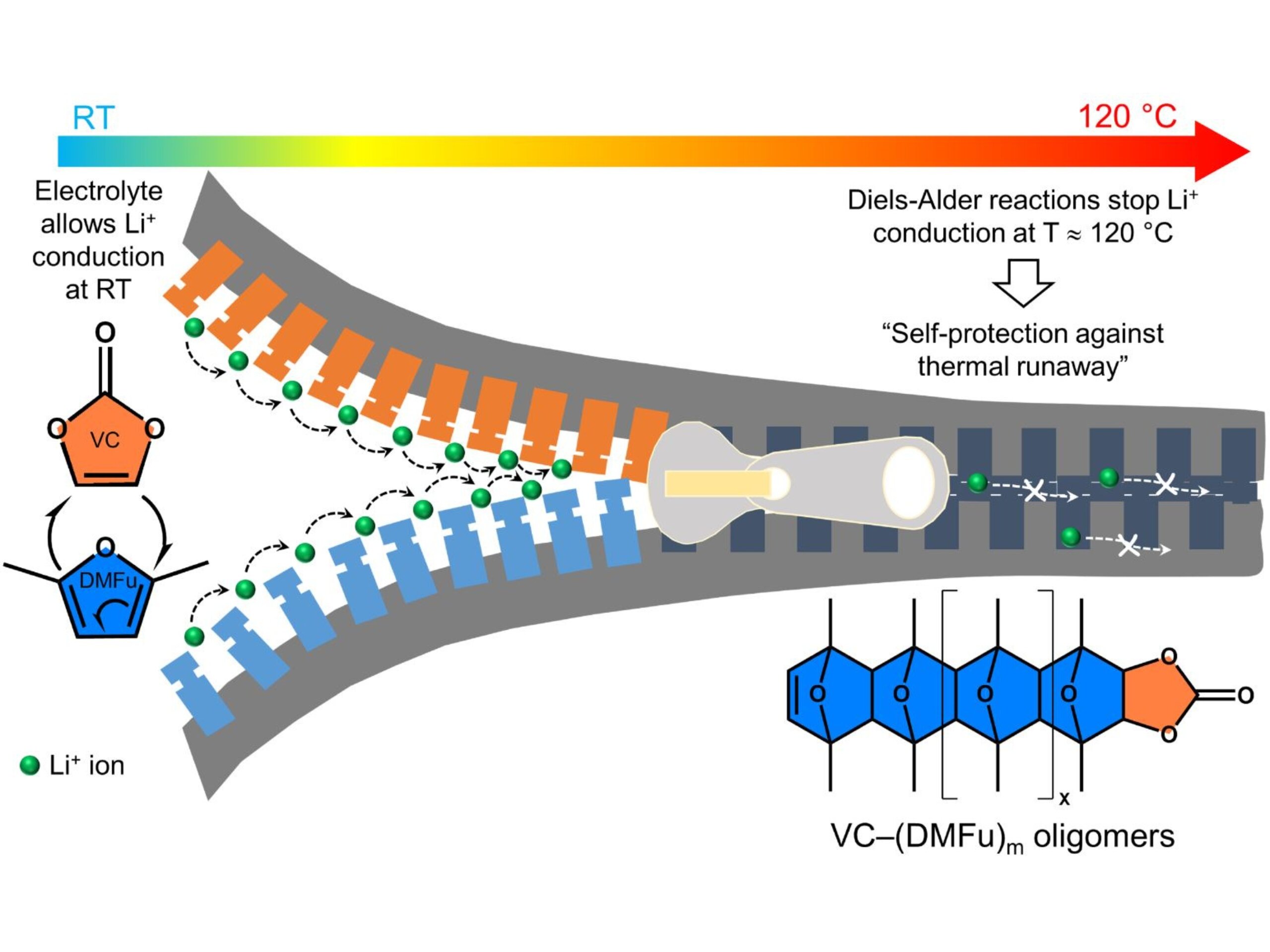
The IMDEA team has engineered a novel electrolyte mixture that responds to temperature in a way that mimics biological reflexes: it reacts immediately, precisely, and predictably. The formulation consists of a lithium salt dissolved in a specially chosen solvent mixture—vinylene carbonate (VC) and 2,5-dimethylfuran (DMFu). At room temperature, this mixture performs just like a standard electrolyte, smoothly conducting lithium ions back and forth between the battery’s anode and cathode during charging and discharging.
But as temperatures climb—particularly above 100°C, a point at which early warning systems become crucial—this smart electrolyte undergoes a radical shift.
According to Dr. Ghosh, “At elevated temperatures, these thermoresponsive electrolytes have been shown to significantly reduce the lithium-ion conductivity of the electrolyte and, at the same time, obstruct the micropores of the separator.”
This dual action acts like an automatic circuit breaker. First, the ionic conductivity plummets, halting the flow of lithium ions that drives battery reactions. Simultaneously, the electrolyte changes its physical behavior, partially clogging the microscopic pores in the separator that lithium ions normally use as highways. This self-induced bottleneck further prevents any reactions from taking place.
The result is a two-step thermal regulation process: at 100°C, the electrolyte begins to reduce performance—what researchers call the “warning phase.” At 120°C, the electrolyte effectively shuts down all activity inside the battery—a “hard stop” that prevents runaway reactions. Unlike traditional separators that wait for failure, this formulation preempts it.
Beyond Fireproofing: Building Intelligence into Battery Design
What makes this research revolutionary isn’t just the idea of safety—it’s the philosophy behind it. Traditional battery safety features are defensive; they’re about containing disasters. The thermoresponsive electrolyte shifts the paradigm to prevention, where the battery actively regulates its own behavior in response to danger.
In other words, we’re seeing the dawn of smart materials in energy storage—components that don’t just serve a single mechanical function but instead sense, interpret, and respond to their environment.
This approach could open new avenues far beyond electric cars or smartphones. Imagine aerospace batteries that safeguard themselves during extreme flight conditions, grid-level energy storage systems that resist catastrophic failures during heatwaves, or wearable electronics that can shut down if they sense risk to the user.
The implications for electric vehicles (EVs), in particular, are profound. Battery fires in EVs, though rare, are high-profile and difficult to extinguish. A thermoresponsive system built directly into the electrolyte would reduce not just the severity of battery failures but the likelihood of them happening in the first place—improving public trust in EV safety and accelerating adoption.
A Marriage of Physics and Engineering
Dr. Ghosh and his colleagues haven’t just mixed chemicals; they’ve designed an integrated system rooted in materials physics, thermodynamics, and chemical kinetics. Achieving such precise control over thermal thresholds is no small feat. It requires understanding how electrolyte molecules interact with lithium salts, how they diffuse through separator membranes, and how their structure changes under thermal stress.
One of the key breakthroughs was identifying vinylene carbonate (VC) and 2,5-dimethylfuran (DMFu) as solvents that could both maintain performance at ambient temperatures and sharply degrade conductivity when heated. Their volatility and phase transition properties were carefully tuned, ensuring the electrolyte remains functional during normal operation but quickly transitions to an inert state under duress.
Moreover, the compatibility of this new electrolyte with existing lithium-ion battery architectures means it could be integrated without wholesale redesign. This is a crucial advantage. Battery innovation often stalls at the commercialization stage because new materials require costly manufacturing changes. IMDEA’s electrolyte is designed with practicality in mind—making it a candidate not just for lab success, but real-world impact.
From Lab to Market: The Road Ahead
Translating smart electrolyte technology into mass production isn’t without hurdles. Electrolyte stability over many charge cycles, long-term storage behavior, and performance across different battery chemistries are all areas that need further investigation. But the early results are promising, and the research has already attracted attention from European battery stakeholders and automotive OEMs.
What makes this innovation particularly exciting is its alignment with the European Green Deal and Battery 2030+ objectives. These EU-led initiatives call for battery solutions that are not only high-performance but sustainable, safe, and scalable. The thermoresponsive electrolyte checks all three boxes. It adds a layer of safety without increasing resource demand. It prevents catastrophic failure without toxic additives or exotic materials. And it could be adapted to a range of next-generation chemistries, including solid-state batteries.
Safety as a Design Principle, Not an Afterthought
The success of the SMARTBATT project is a reminder that safety isn’t just a box to check—it’s a performance metric. Just as we measure batteries by their energy density, cycle life, and charging speed, we must begin to value their ability to protect themselves and their users.
The thermoresponsive electrolyte isn’t a patch or a plug-in. It’s a system-level upgrade, woven into the very architecture of the battery, acting with foresight rather than reaction. This represents a philosophical shift in how we think about technology: one where components are not only efficient but also intelligent and ethical, designed with human and environmental well-being at the core.
A Spark of Genius for a Safer Future
In a world increasingly reliant on battery power, from remote villages powered by solar microgrids to hyper-connected cities pulsing with IoT devices, the stakes are high. The dream of a clean, electric future must be matched by our commitment to making that future safe, reliable, and resilient.
Thanks to Dr. Arnab Ghosh and his team at IMDEA Materials, that dream is one step closer to reality. Their smart electrolyte doesn’t just put out fires—it prevents them. It doesn’t just power devices—it protects lives. And in doing so, it reminds us that the best scientific breakthroughs don’t just push boundaries—they redefine them.
This isn’t just chemistry. It’s foresight, engineered.
Reference: Arnab Ghosh et al, Deciphering a New Electrolyte Formulation for Intelligent Modulation of Thermal Runaway to Improve the Safety of Lithium‐Ion Batteries, Advanced Functional Materials (2025). DOI: 10.1002/adfm.202502761