For someone living with Parkinson’s disease, each step can feel like walking through water, struggling not only with gravity but with their own body’s resistance. Over time, this neurodegenerative disorder wages a quiet war on movement—first slowing it, then stealing it. The once effortless act of walking becomes fragmented: shorter steps, shuffling feet, swinging arms that stiffen and pause as if unsure whether to move at all. Doctors have a name for this haunting alteration: Parkinson’s gait.
It’s more than just a peculiar walk. Parkinson’s gait erodes a patient’s independence. It increases the risk of falls, fractures, hospital visits. But worst of all, it places a constant reminder before patients that their world—once so mobile and fluid—has narrowed. Each sidewalk or hallway becomes a challenge. Each doorway a potential hazard.
While deep brain stimulation (DBS) has transformed care for many Parkinson’s symptoms, especially tremors, rigidity, and bradykinesia (the slowing of movement), its effects on walking have been less reliable. Some patients experience a dramatic improvement in their gait. Others feel little change. For clinicians, this variability has been a frustrating puzzle. Why does it work so well for some, and not for others?
At the heart of this mystery is a lack of tools—reliable, objective tools—to precisely measure gait and its response to DBS. Without them, fine-tuning brain stimulation has felt more like art than science. Until now.
When Engineering Meets Medicine
At the University of California, San Francisco (UCSF), a team of researchers took a radical approach. What if gait dysfunction could be optimized like an engineering system? What if brain stimulation could be mapped, modeled, and personalized using technology?
Led by Dr. Hamid Fekri Azgomi and Dr. Doris Wang, the UCSF team decided to look at the problem differently—not just as neurologists but as systems engineers. They developed a method to observe how different brain stimulation settings affected real-time walking in patients with Parkinson’s. This wasn’t a trial-and-error guesswork game. This was precision medicine infused with machine learning, neuroscience, and a new level of personalization.
The results, published in npj Parkinson’s Disease, offer more than hope—they offer a roadmap for transforming how DBS is used to restore mobility.
Stepping Into the Future: A New Way to Measure Gait
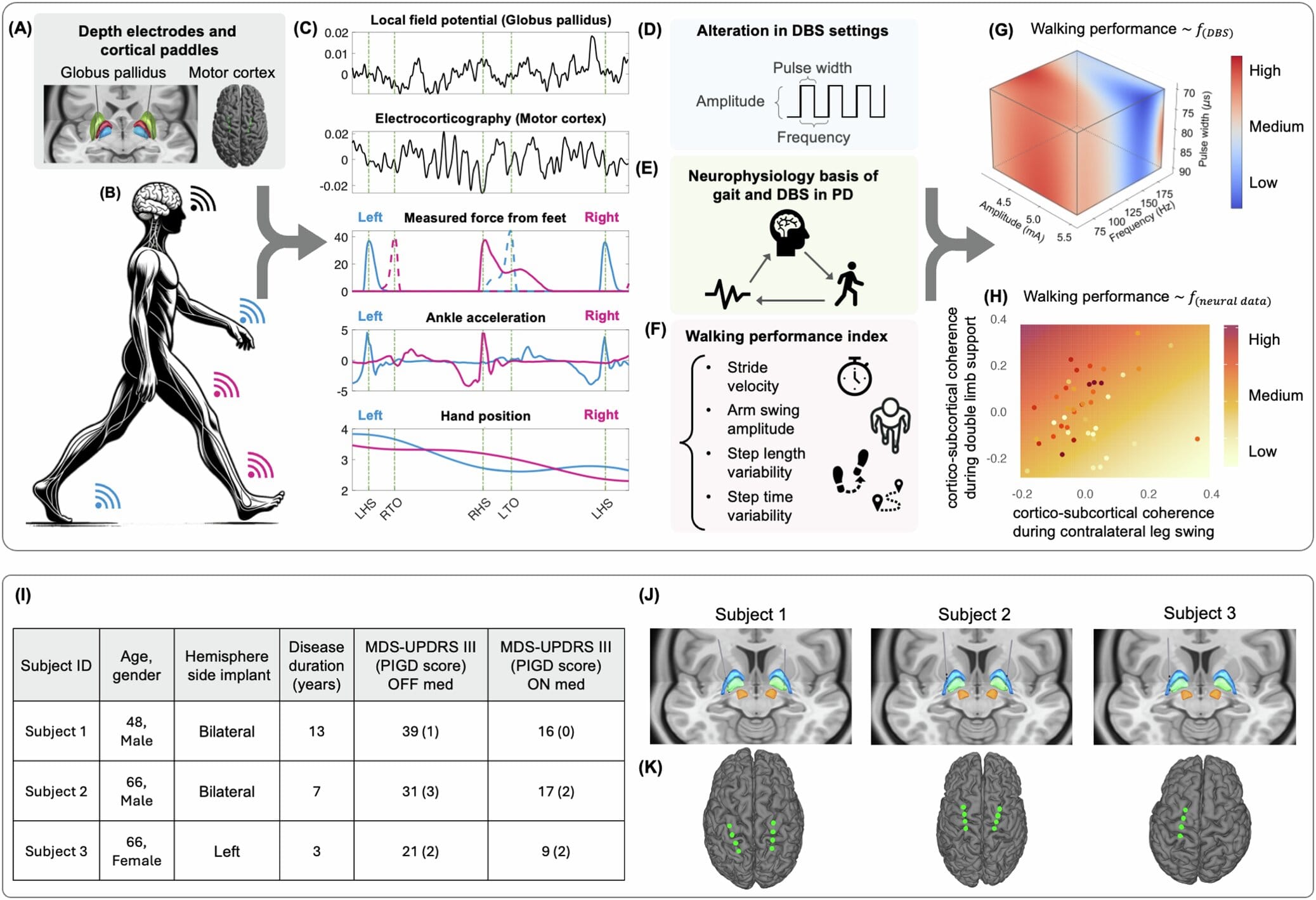
The breakthrough began with a simple but powerful insight: you cannot improve what you do not measure. And in Parkinson’s care, gait—one of the most debilitating symptoms—had no unified metric. So the team built one from the ground up.
They called it the Walking Performance Index (WPI), and it did what previous gait assessments had not. Instead of focusing on just one or two aspects like speed or stride length, the WPI captured a wide spectrum of movement patterns. It measured step length, stride velocity, arm swing, and consistency over time. It was holistic and multidimensional—more like listening to an orchestra than a single instrument.
Each time a patient walked a six-meter loop in the lab, neural activity was recorded in parallel with every movement they made. With each loop and each adjustment to the DBS settings, the researchers gathered data—not just of body movement, but of the brain’s symphony as it attempted to walk.
Then came the next step: turning that data into insight.
Personalized Programming Powered by the Brain’s Own Signals
What the team did next was a powerful fusion of neuroscience and artificial intelligence. They used machine learning to analyze the relationship between brain activity and gait performance. Instead of assuming a one-size-fits-all setting for the DBS devices, they let the data speak for itself.
And it did.
Certain brain patterns, particularly reductions in beta-band brainwave activity within a deep structure known as the globus pallidus, consistently appeared when gait improved. These brainwaves—often overactive in Parkinson’s—are thought to contribute to the freezing and slowness of movement that plague patients. When they quieted down during specific phases of walking, step patterns became smoother, faster, more confident.
By recognizing each patient’s unique neural signature, the researchers could adjust DBS settings in ways that optimized walking. The results were profound. Many patients took longer, more stable steps without sacrificing improvements in tremor or rigidity. It was a delicate dance—preserving what was already working while pushing further into what hadn’t been fully treated before.
This was not just brain stimulation. This was brain communication.
Reclaiming Movement—One Step at a Time
To understand the impact, one must listen to the stories behind the science. A man who once feared falling with every step began walking more freely through his neighborhood. A woman who relied on a walker found herself using it less. These aren’t miracle cures—but they are meaningful changes, the kind that ripple outward into everyday life: walking to the bathroom unaided, navigating stairs without fear, returning to a cherished garden.
Each of these victories is built not on general treatment, but on personalized precision. The settings that work for one patient might not work for another. But by measuring and modeling the brain’s response to movement, clinicians are now equipped to craft DBS therapy that evolves with the individual—no longer fixed in time, but dynamic and responsive.
Dr. Azgomi likened the process to adjusting the strings of an instrument: “We approached the problem as an engineering challenge, aiming to model the relationship between stimulation parameters, brain activity, and walking performance.” And with each adjustment, the brain’s natural rhythm became a little more harmonious.
From Clinical Study to Real-World Innovation
Of course, lab-based success is just the beginning. The real test lies in the broader world—where people walk on uneven sidewalks, climb stairs, and navigate crowded supermarkets. The UCSF team sees this clearly. Their next step is to integrate these discoveries into real-time systems that can adjust DBS on the fly.
Imagine a future where sensors in shoes or belts track your walking patterns and communicate directly with your brain stimulator. When your gait falters, the system adjusts. When your rhythm returns, the stimulation dials back. It’s not science fiction—it’s what these researchers are building toward.
Technologies like wearable motion sensors, smart gait mats, and mobile brain monitoring will allow clinicians to fine-tune therapy not once a year in a clinic, but continuously, in daily life. And when combined with AI, these tools can learn from each patient’s behavior, predicting issues before they become dangerous and tailoring support as the disease evolves.
Smarter Neuromodulation for a Smarter Tomorrow
What makes this work revolutionary is not just its focus on gait, but its philosophy. It recognizes that Parkinson’s disease is not uniform. Every patient is a mosaic of symptoms, responses, and brain activity. Treatments must meet them where they are—not in general terms, but with personalized precision.
For decades, neuromodulation therapies like DBS have operated in black-and-white: a few settings, a few adjustments. But this research opens the door to full-color personalization. It suggests that with the right tools and metrics, clinicians can listen more closely to the brain’s whispers—and respond with exactly the right tune.
“This work not only deepens our understanding of how DBS affects movement,” said senior author Dr. Doris Wang, “but also highlights the promise of personalized neuromodulation for Parkinson’s and other neurological disorders.”
The implications stretch beyond Parkinson’s. Conditions like dystonia, essential tremor, depression, and even chronic pain may one day be treated using similar approaches—tailored, data-driven, and deeply responsive.
Walking Into a Future of Hope
For now, the work continues. More studies, more patients, more refinement. But the path is clearer than ever before.
What began as a mysterious disorder that stole movement step by step is slowly being unraveled by science, compassion, and technology. Thanks to innovative minds and determined hearts, people with Parkinson’s are finding their rhythm again—not just in their brains, but in their lives.
And as they walk forward, so too does the science—toward a future where every step is not feared, but celebrated.
Reference: Hamid Fekri Azgomi et al, Modeling and optimizing deep brain stimulation to enhance gait in Parkinson’s disease: personalized treatment with neurophysiological insights, npj Parkinson’s Disease (2025). DOI: 10.1038/s41531-025-00990-5