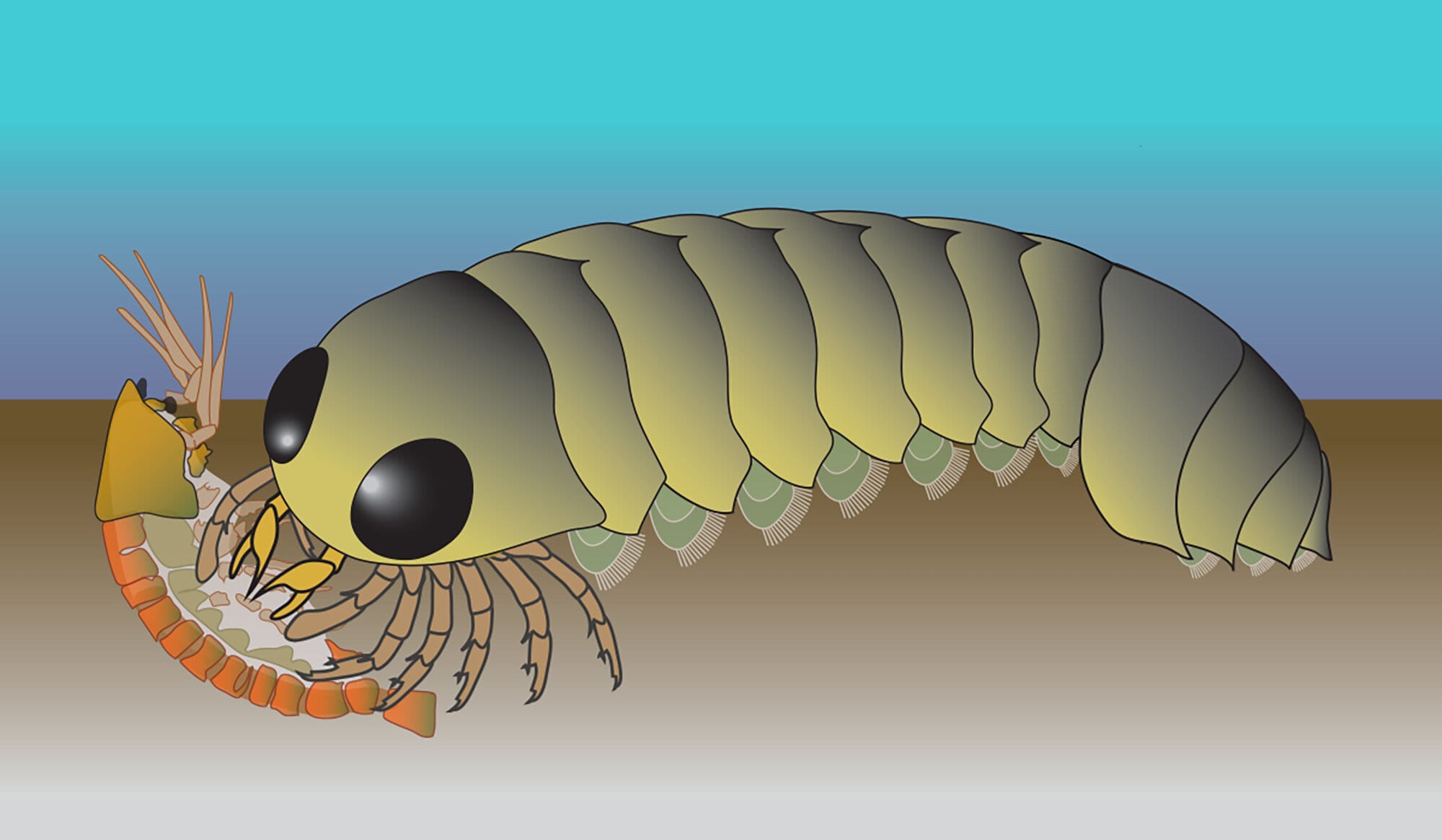
Long before predators stalked dry land, before forests shaded the continents and wings sliced through the air, Earth was ruled by alien seas. In the dark, shifting waters of the Cambrian oceans over 500 million years ago, evolution was composing the first chapters of complex life. There, in sediment-rich shallows, a strange creature known as Mollisonia symmetrica crept along the seafloor—its armored body glinting under filtered light, its movements purposeful, almost stealthy.
For decades, Mollisonia was seen as just another extinct marine arthropod, one of many early animals that helped sketch the outlines of the chelicerate lineage—the group that today includes horseshoe crabs and arachnids like spiders, scorpions, and mites. But a new study, led by neuroscientist Nicholas Strausfeld of the University of Arizona, has shattered this long-held view. Hidden within Mollisonia’s fossilized remains lay an astonishing secret: a preserved brain, exquisitely detailed and unmistakably arachnid.
This fossil brain, its neural highways etched like whispers in stone, suggests a shocking conclusion. Spiders may not have evolved on land as previously believed. Their story may have begun in the ocean.
An Unexpected Legacy Etched in Stone
The fossil in question, unearthed from Cambrian deposits and preserved at the Museum of Comparative Zoology at Harvard University, reveals an organism divided into two familiar sections: a rounded head shield, or carapace, and a segmented trunk ending in a fan-like tail. At first glance, it resembled many Cambrian arthropods—simpler, ancient ancestors of modern crustaceans and horseshoe crabs.
But when Strausfeld examined the fossil under variable lighting and polarization, he noticed something unprecedented. Inside the head region of Mollisonia symmetrica, there were outlines of a neural structure—ganglia arranged in a distinctive radial pattern, five segmental nerve clusters emerging like spokes from a wheel, coordinating a set of paired appendages.
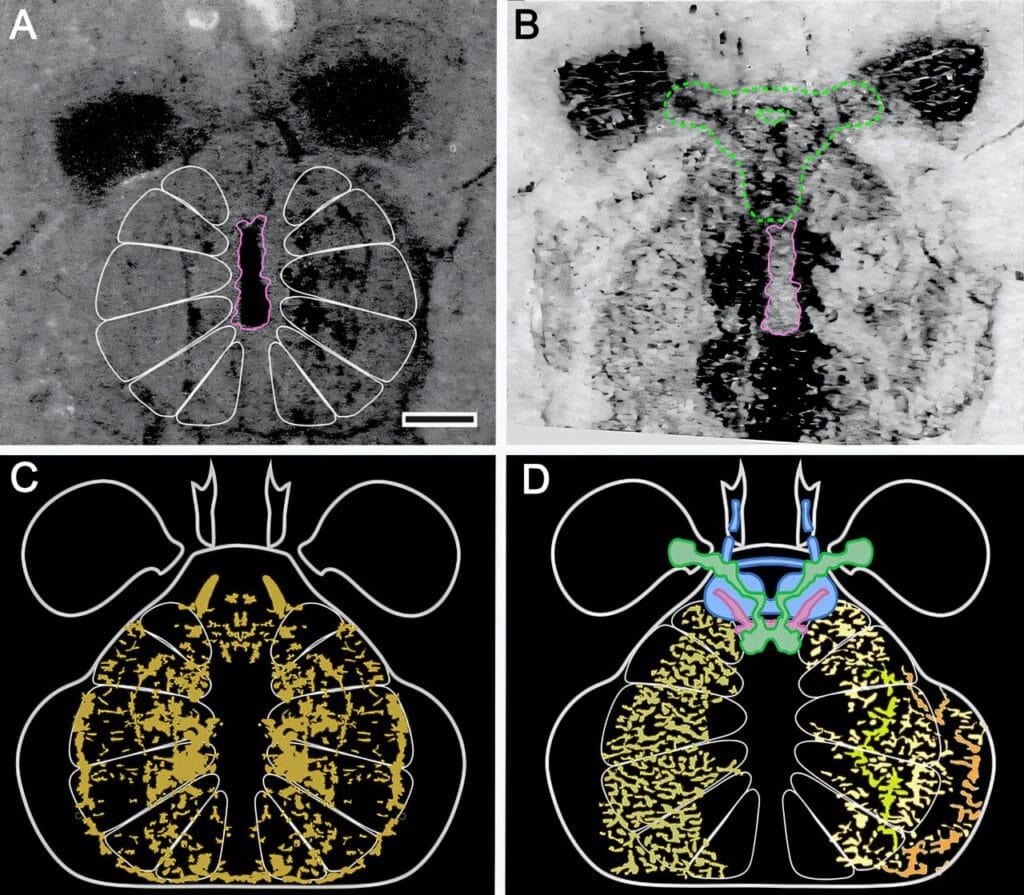
What made this discovery remarkable was how familiar the pattern was. These features didn’t match those found in modern-day crustaceans or even ancient horseshoe crabs. They matched the nervous system of spiders—not just vaguely, but intimately, down to the way the brain extended forward to control a pair of pincer-like appendages resembling spider fangs.
For decades, paleontologists had assumed that arachnids only arose after their ancestors moved onto land. Their fossil record strongly associated them with terrestrial environments, their tough exoskeletons well-suited for life outside the sea. But this tiny marine fossil, no more than a few centimeters in length, told a different story—a story in which the origin of arachnids was aquatic, and their evolutionary adaptations began far earlier than previously thought.
The Reversal That Changed Everything
One of the most puzzling features of Mollisonia’s preserved brain is its reversed architecture. In crustaceans, insects, centipedes, and horseshoe crabs, the neural organization follows a front-to-back sequence that reflects the early ancestral blueprint of segmented invertebrates. But in Mollisonia, as in spiders and scorpions, the sequence was flipped. Neural centers that would normally be found toward the back were shifted to the front—a reversal that might seem bizarre but is now believed to be fundamental to arachnid success.
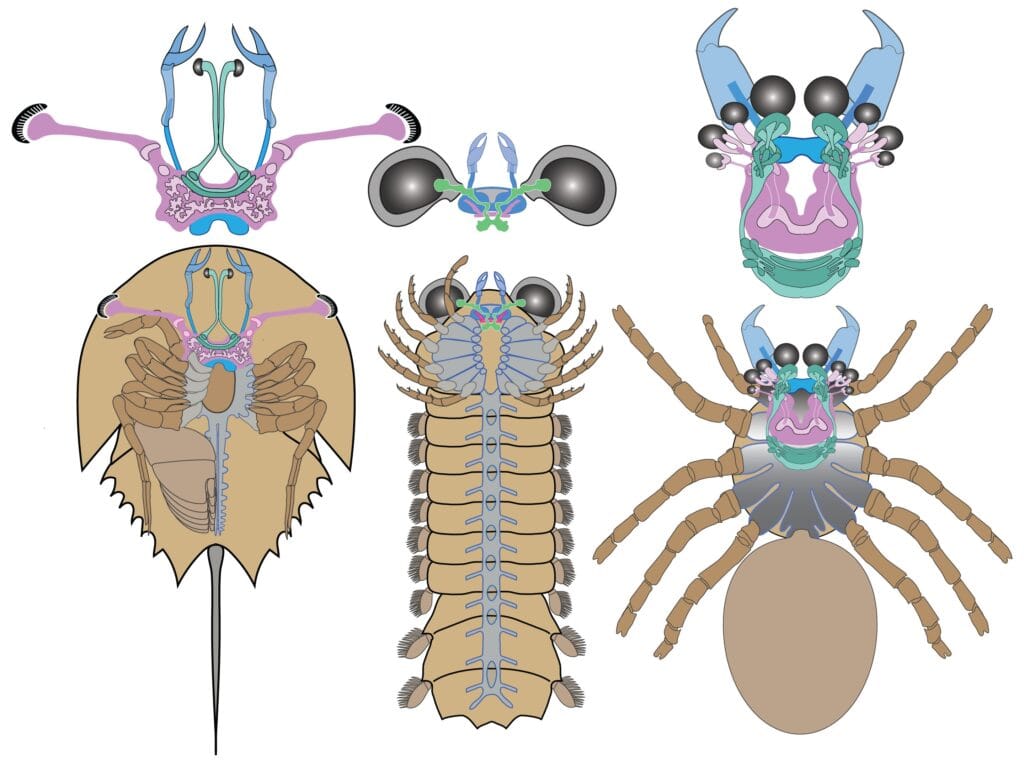
Frank Hirth, a co-author of the study from King’s College London, suggests that this reversal was no accident. It might have provided evolutionary advantages in reaction time, sensory coordination, and predatory precision. In modern spiders, for example, this brain structure likely supports the rapid and silent coordination of limb movements, enabling ambush predation, silk spinning, and highly refined web construction.
This neurological architecture isn’t just different. It’s uniquely arachnid. And finding it fossilized in a Cambrian seabed hints that the brain itself—long hidden from the fossil record—could hold the deepest clues to evolutionary ancestry.
Fangs in the Water: A Predator’s Blueprint
Beyond the overall shape of the nervous system, other anatomical features in Mollisonia reinforce its arachnid identity. Near the front of the head, two short nerves extend to a pair of claw-like appendages—structures reminiscent of chelicerae, the distinctive fang-like mouthparts seen in spiders and their kin. These appendages, capable of grasping or piercing, likely played a central role in feeding and hunting, as they do in modern arachnids.
The fossil also hints at sophisticated segmentation in its trunk and appendages, with musculature and limb coordination that would later serve as the basis for terrestrial locomotion. In the Carboniferous, arachnids would scuttle across forest floors. But in the Cambrian, their ancestors may have already been marine predators, honing the neurological and anatomical features that would later flourish on land.
Strausfeld and his team argue that these adaptations didn’t suddenly emerge in response to a new terrestrial environment. Instead, they may have evolved underwater, perfected in ancient reef flats and tidal channels, where stealth, speed, and agility were just as vital as they are in today’s jungles and deserts.
A Predator Begets Wings
The implications of this research extend beyond arachnids. If early arachnid-like animals were already predatory in the ocean and adapted smoothly to terrestrial life, it changes how we view early land colonization. Strausfeld offers a provocative hypothesis: the emergence of land-dwelling arachnids may have pressured early insects to develop one of their most critical evolutionary traits—wings.
“If you’re being hunted by something fast and venomous,” he suggests, “the ability to fly becomes more than just an advantage. It becomes a necessity.”
This predator-prey arms race may have accelerated the development of insect flight, long seen as one of evolution’s most groundbreaking inventions. And while wings allowed insects to escape their eight-legged hunters, spiders quickly adapted. Their webs followed. Their venom evolved. The chase never ended.
Even today, in the quiet corners of our homes and forests, spiders still catch their airborne prey—remnants of an ancient rivalry that began in the Cambrian sea.
Brains That Define a Clade
To further test their findings, Strausfeld’s team conducted an extensive statistical comparison of 115 neurological and anatomical traits across a wide array of arthropods, extinct and extant. The analysis, performed by co-author David Andrew, revealed that Mollisonia fell as a sister group to modern arachnids. This wasn’t a product of convergent evolution or coincidence. It was a lineage.
These findings challenge the current arthropod family tree and suggest that chelicerate evolution is far more complex than previously understood. Instead of branching neatly into land and sea divisions, the history of these animals appears more tangled—woven through overlapping habitats, shifting ecosystems, and brain architectures that evolved in unexpected directions.
The study also opens up new avenues for exploring the genetics behind this transformation. Since the brain plan is unique to arachnids, researchers believe it may be tied to specific genetic pathways that influence neural growth, limb articulation, and behavior. By identifying these pathways in modern species, scientists can infer how they may have originated in creatures like Mollisonia, giving us a clearer picture of how brains evolved to shape survival.
When Stones Speak, We Listen
In paleontology, soft tissues like brains are the holy grail—rare, delicate, and almost always lost to time. That Mollisonia symmetrica preserved its nervous system at all is a gift of geological fortune. That we now have the tools and insight to interpret it is a triumph of scientific progress.
And what this tiny fossil tells us is not just about spiders or scorpions or Cambrian seas. It’s about the strange and often unpredictable path of evolution. It’s about how form follows function—and how both can surprise us.
Spiders, so familiar in their webs, carry within them the echoes of a seafloor hunter. Their ancestors once crept through ancient waters, their brains already preparing them for the intricate dance of ambush and silk. Their lineage spans oceans and epochs, threading through mass extinctions, continental drift, and the rise of vertebrates.
In the deep stillness of a fossil slab, under the glow of a microscope, those stories come alive again. Not just in bones and shells, but in neurons and nerve bundles, in the soft tissue intelligence that once animated a creature’s every move.
The sea, it seems, was the first stage for arachnid ingenuity.
And Mollisonia—that ancient whisper from a Cambrian tide—is the proof.
Reference: Cambrian origin of the arachnid brain, Current Biology (2025). DOI: 10.1016/j.cub.2025.06.063. www.cell.com/current-biology/f … 0960-9822(25)00822-X