In the silent shallows of coastal mudflats, the sea anemone lives out its quiet life—soft, colorful, tentacled, and largely immobile. To most of us, it’s just another delicate marine creature, swaying with the tides. But to scientists probing the origins of animal life, it has just whispered one of evolution’s deepest secrets.
In a groundbreaking study published in Science Advances, researchers from the University of Vienna have discovered that sea anemones, despite their simple, flower-like appearance, use an ancient genetic trick to shape their bodies—one that’s also found in animals as complex and different as frogs, sea urchins, and humans.
This molecular choreography, known as BMP shuttling, may be more than just a developmental tool—it could be the key to understanding how the blueprint for bilateral symmetry, the basic body plan of most animals, first emerged on Earth over 600 million years ago.
A Tale of Two Body Plans
Most animals we know—dogs, birds, insects, whales, even people—are bilaterians. That is, they have a front and back, a top and bottom, and a left and right. This arrangement is called bilateral symmetry, and it defines the vast majority of the animal kingdom.
Then there are the cnidarians—sea anemones, jellyfish, and corals. They’re usually thought of as radially symmetrical, like a pizza or a wagon wheel, with body parts arranged around a central axis. Jellyfish are the poster child for this. But sea anemones? They’ve been keeping a secret.
“Although sea anemones look radially symmetric, when you look closer—at their genes and then later at their adult anatomy—you see bilateral symmetry,” says David Mörsdorf, the study’s lead author and a postdoctoral researcher at the University of Vienna’s Department of Neurosciences and Developmental Biology.
This raises a profound question in evolutionary biology: did bilateral symmetry evolve once in a common ancestor of cnidarians and bilaterians, or did it arise independently, twice?
To answer it, Mörsdorf and his colleagues turned to the early stages of life itself—embryonic development—and a tiny sea anemone called Nematostella vectensis, the unassuming star of this evolutionary detective story.
The Blueprint Within
In bilaterian animals, early in development, embryonic cells must figure out where they are in the body-to-be. Some will become brain, others skin, others spine or gut. To organize this cellular map, the body uses chemical signals—molecular gradients—that tell each cell what fate to adopt based on its position.
One such system uses Bone Morphogenetic Proteins (BMPs)—a kind of biochemical GPS that helps shape the body’s top-to-bottom, or back-to-belly, axis. In bilaterians, BMPs are controlled by a partner protein called Chordin, which binds to BMPs and regulates where they’re active.
Sometimes Chordin simply blocks BMP activity locally. But in many animals—frogs, flies, and sea urchins included—Chordin doesn’t just inhibit. It shuttles BMPs across the embryo, relocating them to different regions. This dynamic process, called BMP shuttling, allows precise gradients to form, guiding the embryonic landscape.
But whether this sophisticated mechanism existed before bilaterians—perhaps in the ancient ancestor they shared with cnidarians—was unknown. Until now.
Experimenting with Embryos
To test whether Nematostella uses BMP shuttling, the researchers genetically manipulated its embryos. First, they blocked the production of Chordin entirely. Without Chordin, BMP signaling ground to a halt, and the sea anemone’s secondary body axis—essential for bilateral structure—failed to form. That meant Chordin was essential.
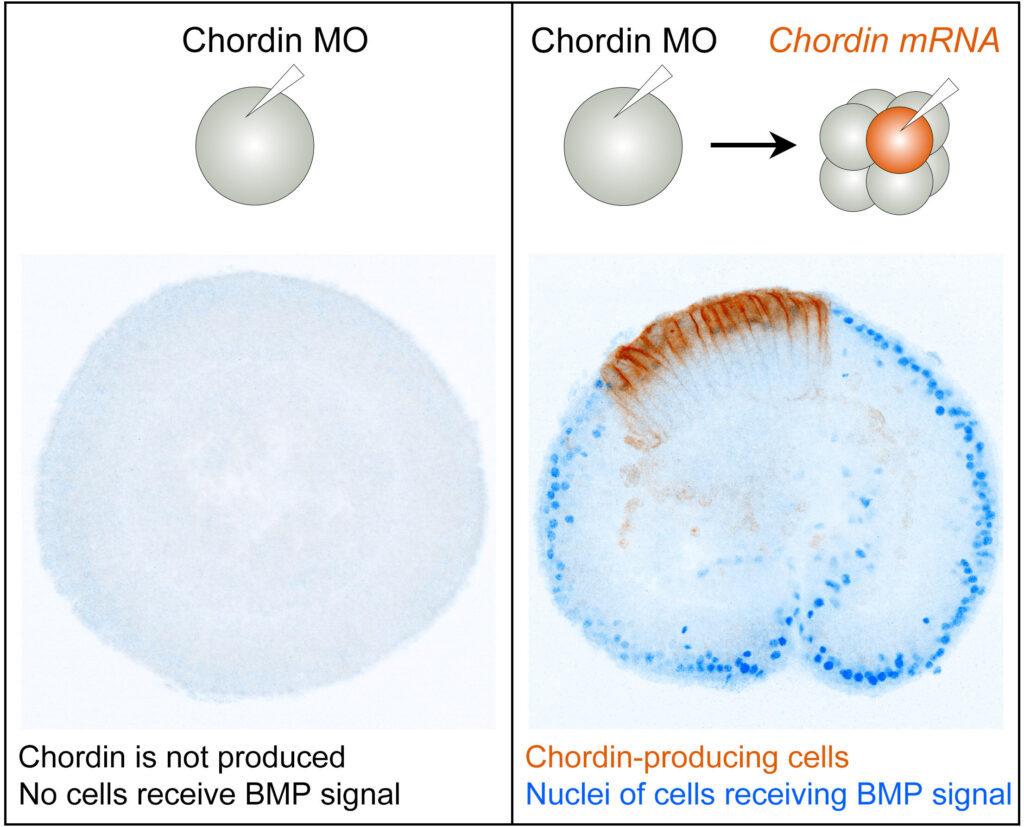
Next, they reintroduced Chordin into only one side of the embryo to see if development could resume. It did—but how?
To tease apart the mystery, they created two versions of the Chordin protein. One was membrane-bound and immobile, acting only where it was inserted. The other was free-floating and diffusible, capable of moving across the embryo.
If Chordin only blocked BMPs where it appeared, then both versions should have worked equally. But only the diffusible version restored BMP signaling at a distance. That meant it wasn’t just blocking—it was transporting. Chordin was actively shuttling BMPs to shape the embryo’s axis.
“The result was crystal clear,” Mörsdorf says. “The sea anemone uses BMP shuttling just like bilaterian animals do. And that tells us something truly remarkable—it’s not a modern innovation. It’s ancient.”
A Shared Ancestry Etched in Molecules
What this suggests is that BMP shuttling—previously thought to possibly have evolved multiple times in different animal groups—may have originated long before complex animals diversified. It could have been present in the last common ancestor of cnidarians and bilaterians, more than 600 million years ago.
That’s like discovering a handwritten instruction manual used by vastly different civilizations on different continents—and realizing they all got it from the same long-lost source.
“Not all bilaterians use BMP shuttling,” Mörsdorf explains. “Frogs do. Fish don’t. But we see it popping up again and again in very distantly related animals. And now we’ve seen it in sea anemones. That gives us strong reason to believe it’s an ancestral mechanism.”
Grigory Genikhovich, senior author and head of the research group, adds: “We might never be able to exclude the possibility that bilaterians and bilaterally symmetric cnidarians evolved their body plans independently. But if their last common ancestor had a bilateral body—and used Chordin to shuttle BMPs—then the roots of our own body plans go much deeper than we thought.”
A New View of the Tree of Life
This discovery forces scientists to reconsider the early evolution of animals—not as a clean, radial-to-bilateral split, but as a more complex story of ancient developmental strategies, inherited and repurposed across deep time.
It’s not just a matter of sea anemones and humans sharing some genetic quirks. It’s about understanding how multicellular life organized itself at its very beginnings—how a collection of cells first found direction, shape, and purpose.
Evolution, it turns out, is not always about invention. Sometimes, it’s about preservation.
And sometimes, a humble sea anemone—rooted to the seafloor, arms waving in the tide—can help us see how the great body plans of life were shaped, not just by time, but by a whisper from a shared past.
Reference: David Mörsdorf et al, Chordin-mediated BMP shuttling patterns the secondary body axis in a cnidarian, Science Advances (2025). DOI: 10.1126/sciadv.adu6347