Beneath the thresholds of our naked eyes, in realms untouched by sunlight or breath, lies a hidden world teeming with life’s imitators. Viruses are neither truly alive nor fully inert. They do not breathe, do not grow, do not eat. Yet they move, they multiply, they evolve—and they leave deep scars on human history. These invisible entities are some of biology’s most paradoxical creations: biological ghosts wrapped in protein coats, slipping quietly into the machinery of living cells, commandeering their systems like expert saboteurs. And yet, without them, evolution itself might never have found the same paths.
Understanding how viruses work requires more than memorizing facts. It means peering into the boundaries of what defines life, infection, and immunity. It means confronting something disturbingly alien, yet strangely beautiful, in its brutal efficiency.
A Shadow on the Edge of Life
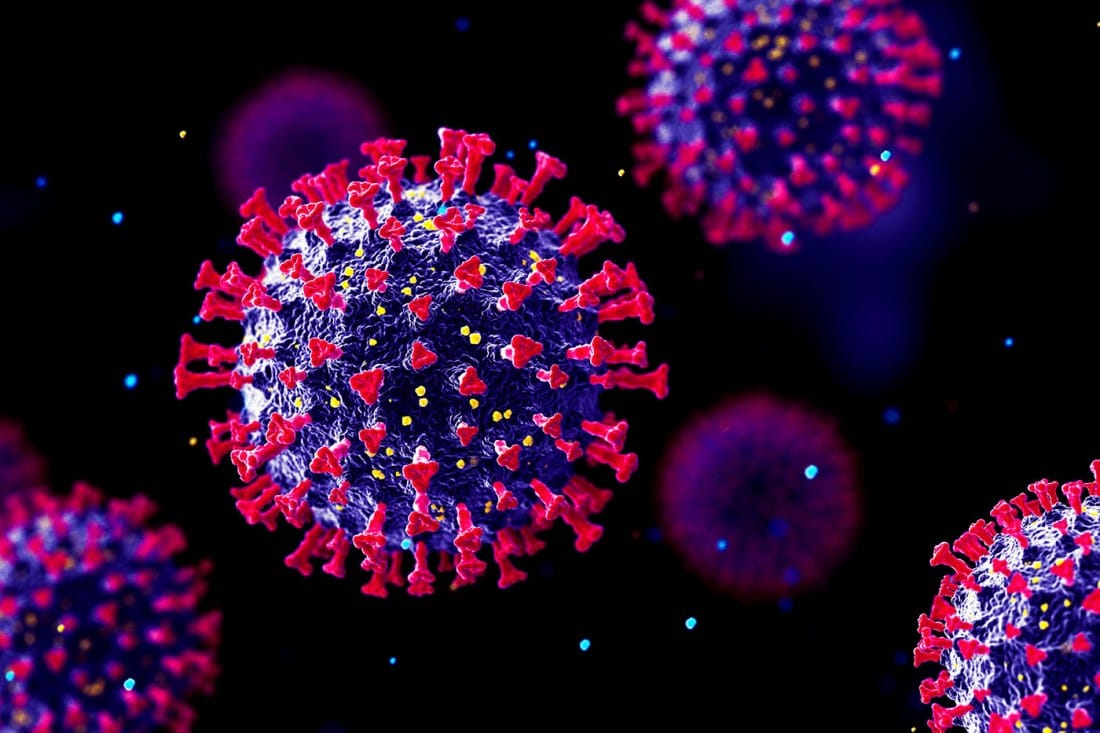
What exactly is a virus? Biologically speaking, a virus is an infectious particle made up of genetic material—either DNA or RNA—encased in a protein shell known as a capsid. Some viruses are further cloaked in a lipid envelope derived from their host cell’s membrane, giving them a ghost-like appearance under the microscope.
But what separates viruses from living organisms is stark: viruses cannot reproduce on their own. They have no cellular structures, no metabolism, and no means to grow or divide. They exist in a kind of suspended animation outside a host, like a seed waiting for rain. Only upon entering a suitable living cell do they awaken, unfurling their genetic code and forcing the host to read it.
This blurry line between the living and the inert is what makes viruses so hauntingly fascinating. They are more than passive invaders. They are code—biological software seeking out hardware. And once that match is found, the consequences can range from mild inconvenience to global catastrophe.
The Anatomy of a Viral Intruder
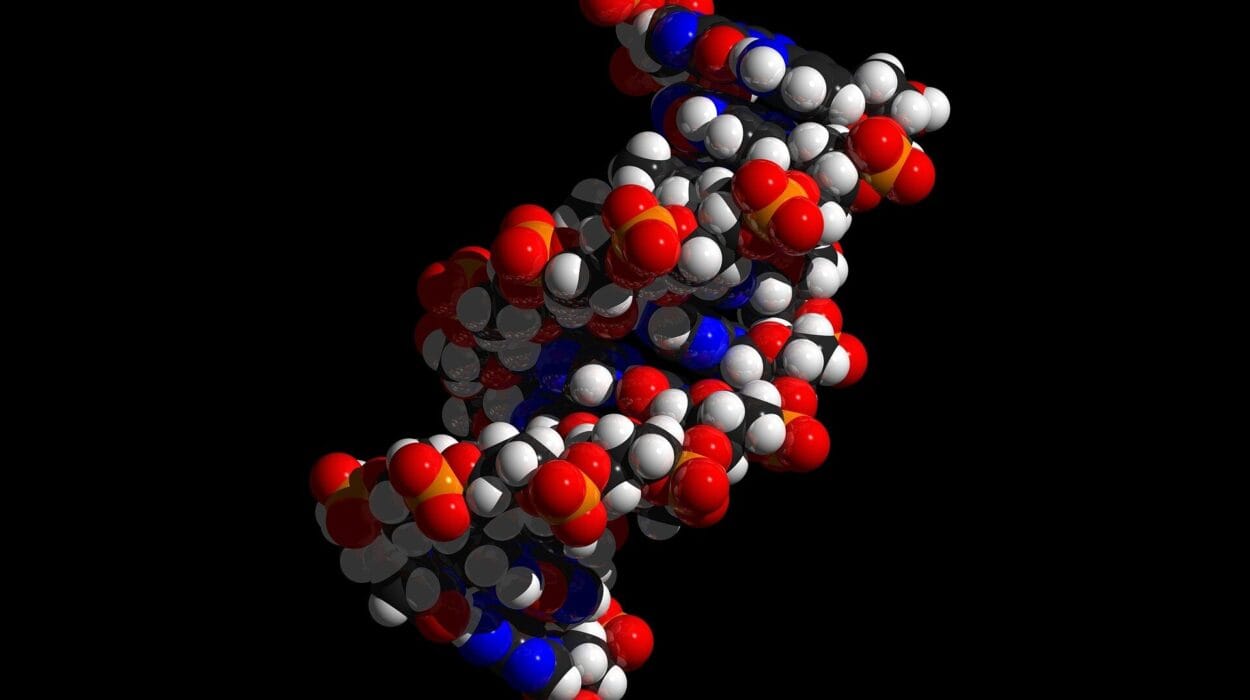
Though viruses vary dramatically in shape, size, and complexity, their basic structure is surprisingly elegant. At their core lies the viral genome, a thread of nucleic acid that encodes everything the virus needs to replicate—once inside a host. Some viruses use double-stranded DNA like we do; others wield single-stranded RNA. Some even carry both, or flip between forms during their life cycles.
Surrounding the genome is the capsid, a protective protein shell often shaped with geometric precision. Icosahedral viruses like adenovirus form near-perfect twenty-sided structures. Others, like influenza, take on more spherical or filamentous shapes. And still others, like the bacteriophage that infects bacteria, look like alien spiders—complex, mechanical, and terrifyingly purposeful.
Enveloped viruses like HIV and SARS-CoV-2 carry an additional layer: a fatty membrane stolen from their host’s own cells. Embedded in this envelope are spike proteins—molecular keys that allow the virus to bind to and enter target cells with surgical precision. These spikes are often the first—and sometimes only—thing our immune system can “see,” making them both the virus’s weapon and its greatest vulnerability.
The Art of Infiltration
A virus’s mission is simple: find a host, enter it, and replicate. But the execution is a masterclass in molecular deception.
The journey begins when a virus comes into contact with a susceptible host cell. Through a process known as “attachment,” the viral surface proteins lock onto specific receptors on the cell’s surface—like a key fitting into a highly selective lock. This is not a casual relationship. If the key doesn’t fit, the virus cannot enter. This specificity is what determines a virus’s host range—whether it infects humans, birds, bats, or only certain cells within those organisms.
Once bound, the virus enters the cell either by fusing its membrane with the host’s or being swallowed through endocytosis, a cellular process usually reserved for nutrients. Inside, the virus sheds its outer shell and releases its genetic payload into the cell’s interior. Now the hijacking begins.
The host cell, oblivious to the betrayal, reads the viral genome as if it were its own instructions. It begins manufacturing viral proteins, assembling new viruses piece by piece. The cell’s own machinery—its ribosomes, enzymes, and energy sources—are commandeered, repurposed to churn out the invader’s progeny.
In the final stage, the newly minted viruses exit the cell. Some do so violently, bursting the cell open in a lytic explosion. Others bud off more subtly, cloaked in pieces of the cell membrane that will now serve as their camouflage. Either way, the host cell is often left dead or damaged, while the new viruses go on to infect their neighbors.
Mutation: The Engine of Viral Evolution
Viruses are masters of adaptation, and their secret weapon is mutation. Unlike human DNA, which is carefully proofread and repaired during replication, viral RNA is often copied sloppily and without correction. Each error—a missing base, a swapped letter—creates a slightly different version of the virus.
Most mutations are neutral or harmful to the virus. But occasionally, one creates an advantage: resistance to a drug, better binding to a host receptor, escape from immune detection. These rare survivors become the new dominant strain, shaping the virus’s evolution in real time.
This is why viruses like influenza change so rapidly, requiring new vaccines each year. It’s also how new strains of a virus—like variants of SARS-CoV-2—emerge and sweep through populations. Mutation is a dice roll, but in the numbers game of viral replication, the odds always favor change.
Recombination adds another twist. When two viruses infect the same cell, they can exchange genetic segments, creating chimeras with traits from both parents. This is one suspected origin of pandemic influenza strains—when bird and human flu viruses mix inside pigs, for example.
Viral evolution is relentless, efficient, and utterly without intent. It is not malicious. It simply is. But its consequences for life on Earth have been profound.
The Immune System Strikes Back
For every viral offense, evolution has crafted a cellular defense. Our immune system is a multi-layered fortress, and its war with viruses is one of biology’s longest-running arms races.
The first line of defense is the innate immune system. When a virus invades, cells release alarm signals—cytokines and interferons—that trigger inflammation and call immune cells to the site. Specialized sentinels like dendritic cells engulf viral debris and present its parts to the adaptive immune system—our second, more sophisticated line of defense.
Adaptive immunity is a symphony of precision. B cells produce antibodies that can bind to viral spikes, blocking their entry into cells. T cells, meanwhile, patrol for infected cells and destroy them before the virus can spread. Once the invader is cleared, memory cells remain—guardians who can respond more rapidly if the virus returns.
Vaccines exploit this memory. By exposing the immune system to a harmless form of the virus—dead, weakened, or fragmented—we train our defenses in advance. Then, when the real virus shows up, we are ready.
But viruses fight back. Some mutate to escape recognition. Others, like HIV, target the immune system itself. The race never ends. It is a conflict measured not in battles, but in generations.
Viruses That Heal and Build
Not all viruses are villains. In fact, many are unsung architects of life as we know it. Some scientists even argue that without viruses, complex life might never have evolved.
Viral infections can drive genetic innovation. A large portion of the human genome—over 8%—is made of ancient viral sequences that became embedded in our DNA. Some of these viral remnants have been repurposed for vital functions, including pregnancy. The protein syncytin, which helps the placenta form, originated in a retrovirus millions of years ago.
Viruses can also be allies in medicine. Bacteriophages—viruses that infect bacteria—are being used to treat antibiotic-resistant infections. Gene therapy techniques often use modified viruses to deliver healthy genes into patient cells. Even cancer treatments are being developed using viruses engineered to attack tumors while sparing healthy tissue.
In ecology, viruses regulate populations and nutrient cycles, particularly in oceans where they kill billions of bacteria daily, releasing organic matter that feeds other organisms.
The truth is, viruses are part of the tapestry of life. They shape it, end it, and sometimes save it. Their story is interwoven with ours.
The Rise of Pandemic Potential
Despite our growing knowledge, viruses still wield the power to upend civilizations. In recent decades, viral outbreaks have tested our preparedness and our humanity. Ebola, Zika, SARS, MERS, and most recently, COVID-19, have reminded the world that microscopic agents can bring global systems to their knees.
What makes viruses so dangerous is not just their biology but their ecology. Deforestation, climate change, and global travel bring humans into closer contact with animal reservoirs. Many viruses that infect humans today—HIV from chimpanzees, influenza from birds and pigs, coronaviruses from bats—began in other species.
These “zoonotic spillovers” are not freak accidents. They are symptoms of a changing world. The more we disturb natural habitats, the more likely it is that new viruses will emerge—and that old ones will return with renewed force.
Pandemics are not just medical crises. They are social, economic, and psychological ones. They reveal fault lines in our systems: inequality, misinformation, distrust. And they challenge us to find not just cures, but compassion.
The Future of Viral Understanding
We now possess tools our ancestors could scarcely imagine. We can sequence a viral genome in hours. We can model its proteins in supercomputers. We can design mRNA vaccines in weeks. But science is not a finish line—it is a journey of perpetual humility.
The next frontier lies in predicting and preventing outbreaks, developing universal vaccines, and perhaps one day eradicating certain viral diseases altogether. Advances in nanotechnology, synthetic biology, and AI may give us the edge we need. But the question remains: will we act in time?
Education and global cooperation are just as vital as science. No vaccine works if people don’t take it. No breakthrough matters if it’s locked behind patent walls. In fighting viruses, we are only as strong as our weakest link.
A Dance That Never Ends
Viruses are not evil. They have no desires, no agenda. They are nature’s purest expression of replication and adaptation. They are sculptors of genomes, shapers of history, and quiet reminders of life’s fragility.
In understanding how viruses work, we uncover more than infection—we uncover connection. Between molecules and mortality. Between the individual and the ecosystem. Between the past encoded in our DNA and the future we hope to write.
And perhaps, in facing the smallest of foes, we rediscover the strength of being human.