Every sensation you feel—whether the sharp sting of pain, the pleasurable rush of taste, or the anxious flutter of stress—is orchestrated by an invisible ensemble of molecular switches. These tiny conductors, known as G protein-coupled receptors (GPCRs), quietly govern the body’s essential responses. Nestled in cell membranes like microscopic sentinels, they are the gatekeepers between the outside world and our internal cellular machinery.
GPCRs are anything but rare. In fact, they represent the largest family of membrane proteins in the human genome. They are stunningly diverse, sensitive to a wide variety of signals, and—most importantly—they’re a pharmacological goldmine. Approximately one-third of all approved drugs owe their effectiveness to their ability to manipulate GPCRs. Painkillers, antidepressants, heart medications, even semaglutide (used in diabetes and obesity treatment)—all of them depend on our ability to harness these molecular switches.
And yet, until recently, one critical mystery remained: How exactly do GPCRs function at a molecular level?
Peering Inside the Machine: A New Molecular GPS
For decades, scientists have struggled to capture the dynamic choreography of GPCRs. The receptors don’t simply flick from “on” to “off” like a light switch. They exist in a shifting cloud of conformations, subtly changing shape in response to molecules called ligands—everything from hormones and neurotransmitters to pharmaceutical drugs.
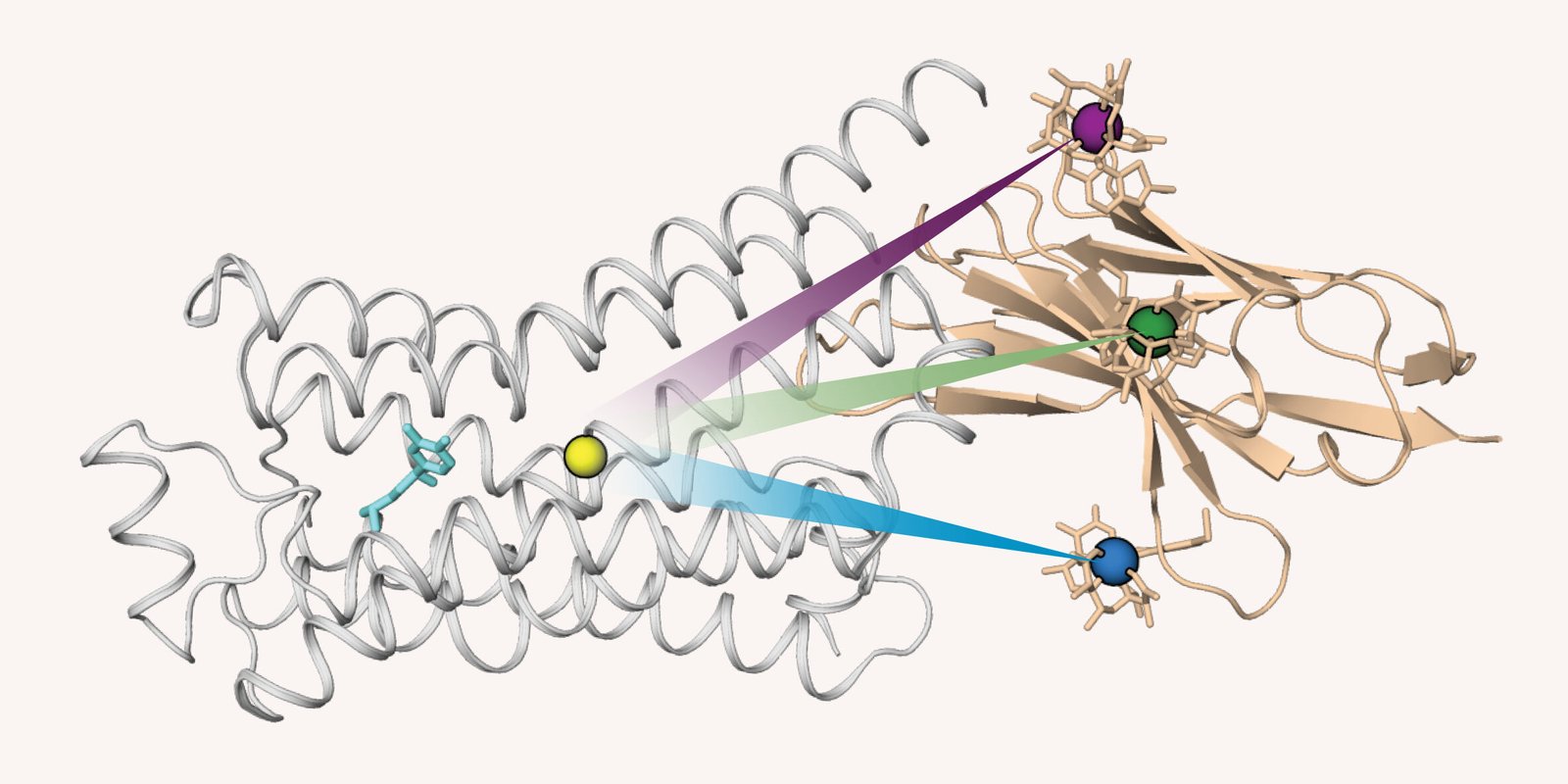
Now, a research team at the University of Basel, led by Dr. Fengjie Wu and Prof. Stephan Grzesiek, has cracked open the black box. In a breakthrough published in Science, they have developed a technique inspired by the global positioning system—a “GPS” for molecules, based on nuclear magnetic resonance (NMR) spectroscopy.
Their innovation, dubbed GPS NMR, allows researchers to pinpoint the position of specific atoms within the GPCR protein and observe how these atoms move when the receptor is activated. “It’s like watching the gears inside a Swiss watch,” says Wu. “But instead of gears, we’re tracking atoms. And instead of time, we’re watching biology unfold.”
Why GPCRs Matter: The Molecular Nexus of Medicine
To grasp the importance of this discovery, consider what GPCRs do. Embedded in the oily membranes that wrap every human cell, GPCRs are the first responders to external stimuli. When a ligand binds to the receptor’s outer surface, it causes a shape-shift that sends a signal inside the cell, usually by activating a G protein. This in turn triggers a cascade of intracellular events: releasing hormones, modulating neuron activity, relaxing blood vessels, or changing the beat of your heart.
Because of their central role, GPCRs are drug targets of extraordinary value. Beta-blockers calm the heart by targeting one class of GPCRs. Opioids dull pain by binding to another. In metabolic diseases, certain GPCRs modulate hunger and insulin sensitivity. The better we understand these receptors, the more precisely we can design drugs that do what we want—without the side effects we don’t.
Zooming In: The β1-Adrenergic Receptor in Focus
The Basel team focused on one particularly important GPCR: the β1-adrenergic receptor, a critical player in the human cardiovascular system. This receptor helps regulate heart rate and blood pressure by responding to adrenaline-like signals. It’s also the primary target of beta-blockers, which are prescribed to millions of people around the world to treat hypertension, heart failure, and arrhythmias.
By applying GPS NMR, the scientists could track the behavior of over 100 specific atomic sites within the β1 receptor. They monitored how these sites moved and changed configuration in response to different drugs—including isoprenaline, a receptor activator (agonist), and beta-blockers, which inhibit activation.
What they found was astonishing: the receptor doesn’t just flip between “off” and “on.” Instead, it cycles through a dynamic spectrum of states—inactive, partially active, and fully active. These states exist in equilibrium, and the presence of specific ligands nudges the receptor toward one side of the spectrum.
“Before, we imagined GPCRs as simple switches,” says Wu. “Now we know they are more like dimmer knobs—finely tuned and constantly adjusting.”
The Central Microswitch: Nature’s Hidden Control Panel
One of the most revelatory findings of the study was the identification of a central “microswitch” within the receptor’s structure. This tiny cluster of atoms functions like a pivot point, controlling how the entire receptor transitions between states. The microswitch is highly conserved across different GPCRs, meaning that evolution has preserved it through countless iterations of life—likely because it’s essential to the receptor’s function.
Even more intriguing, the researchers found that subtle chemical tweaks—tweaks as small as a single atomic change—could dramatically alter the receptor’s behavior. This opens the door to a tantalizing possibility: that next-generation drugs could be designed not just to “turn on” or “turn off” GPCRs, but to fine-tune their response, like adjusting the tone of a musical instrument.
“This gives us a kind of molecular map,” explains Grzesiek. “It tells us where to apply pressure if we want the receptor to behave a certain way.”
From Static Structures to Living Machines
Historically, much of what scientists knew about GPCRs came from static snapshots—X-ray crystallography images that froze the receptors in place. These were useful for understanding their general shape, but they couldn’t capture the motion that defines function.
GPS NMR changes that paradigm. It transforms our understanding of GPCRs from rigid architecture to living machines, constantly in motion and responsive to subtle cues. By bridging the gap between structure and function, the Basel researchers have illuminated one of biology’s most elusive dynamics.
“After 20 years of effort, we can finally see these fine details,” says Grzesiek. “We can watch, almost in real-time, as a drug molecule binds and the receptor changes shape.”
The Road to Smarter, Safer Drugs
This research arrives at a critical time. While GPCR-targeting drugs have transformed modern medicine, they can also cause significant side effects, especially when they indiscriminately activate or block a receptor across different tissues.
By understanding the nuanced spectrum of receptor states, drug developers can now design ligands that push the receptor just enough—activating beneficial pathways while avoiding harmful ones. This approach, known as biased signaling, has emerged as one of the most promising frontiers in pharmacology.
For example, a drug could be crafted to trigger a GPCR’s anti-inflammatory pathway without activating its pain-related pathway—or vice versa. That kind of precision is only possible when you understand the receptor’s full range of behavior, down to the atomic level.
The Future of Molecular Tracking
Beyond GPCRs, the implications of GPS NMR extend to nearly any dynamic protein embedded in cell membranes. Ion channels, transporters, even viral proteins like those in coronaviruses—all could, in theory, be explored using this technique.
Moreover, GPS NMR offers a tool not just for drug development, but for basic biology. It lets researchers see how a protein “breathes,” how it adapts to pressure, temperature, and chemical stress. It provides a window into the moving life of molecules—a field once limited to educated guesses and artistic renderings.
Molecular Precision in a Post-Genomic World
We live in an age where the human genome has been sequenced, where gene editing is common, and where artificial intelligence helps design new drugs. And yet, without understanding how proteins actually move—how they respond to molecular contact, how they shift and signal—we remain blind to some of biology’s most fundamental mechanisms.
The GPS NMR approach pioneered by the University of Basel researchers is a leap forward. It transforms our view of GPCRs from passive receivers to responsive, dynamic, and tunable machines. And with this new knowledge comes the power to engineer better therapies, guided by the rhythms of atomic movement.
“Biology is not static,” Wu reflects. “It dances. And now, for the first time, we can follow the steps.”
Reference: Feng-Jie Wu et al, Activation dynamics traced through a G protein coupled receptor by 81 1H-15N NMR probes, Science (2025). DOI: 10.1126/science.adq9106. www.science.org/doi/10.1126/science.adq9106