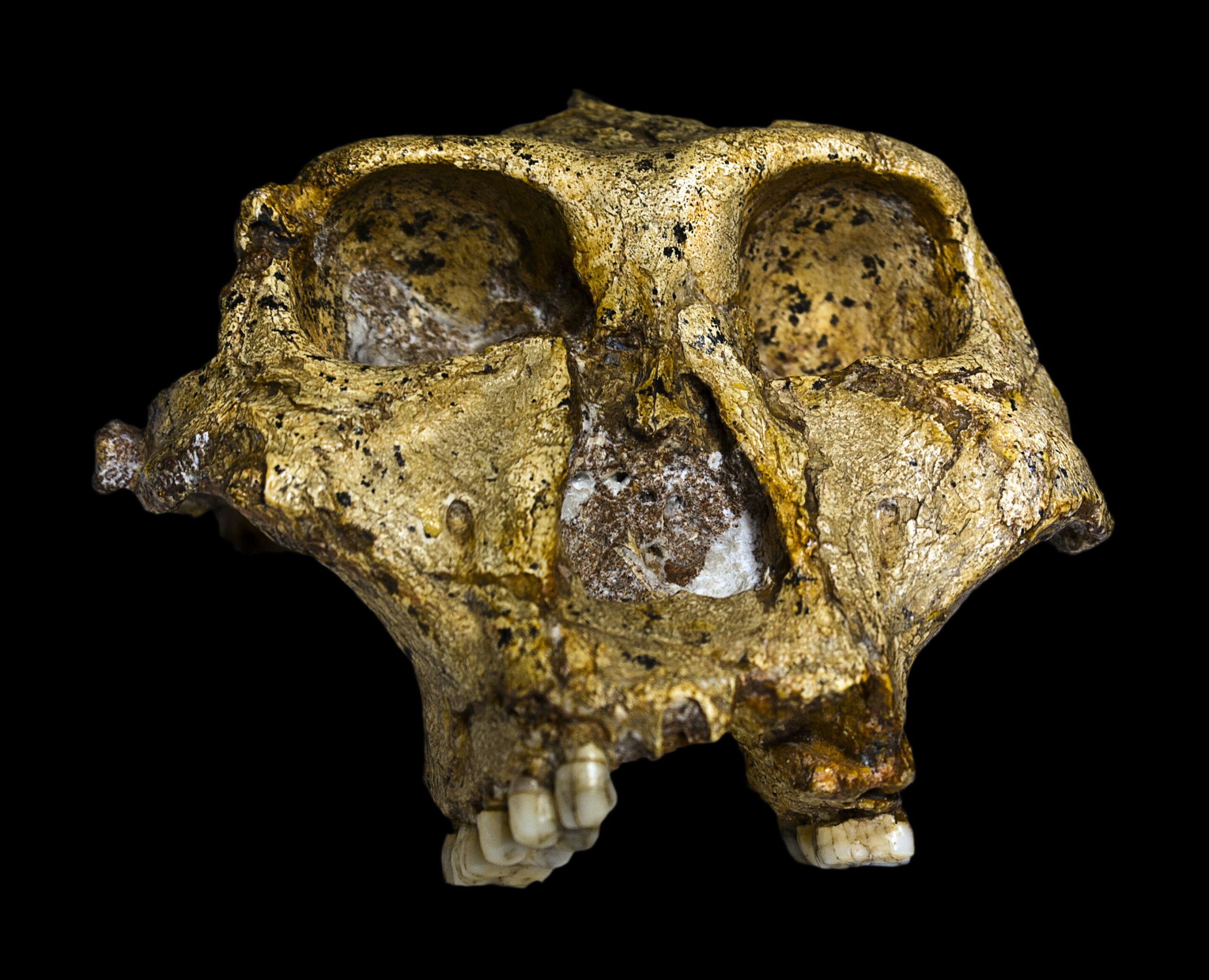
In the cool limestone shadows of South Africa’s Swartkrans cave, a new kind of time traveler is whispering stories—not through bones or tools, but through the microscopic proteins locked inside fossilized tooth enamel. These ancient remnants, once anchored in the jaws of Paranthropus robustus, an enigmatic hominin species that roamed the earth nearly two million years ago, are now revealing secrets never before accessible. And they’re doing so in a most unexpected way: through a discipline at the crossroads of evolutionary biology, molecular archaeology, and paleoanthropology.
In a landmark study published in Science, a multidisciplinary team of anthropologists, evolutionary theorists, and molecular biologists has cracked open a new chapter in the story of human origins. By sequencing enamel proteins from four Paranthropus robustus teeth—dating to between 1.8 and 2.2 million years ago—they’ve been able to determine both the sex and subtle genetic signatures of individuals whose species has long defied easy classification. What they’ve uncovered is rewriting assumptions about gender, group identity, and even how we draw evolutionary boundaries in our ancient family tree.
The Paradox of Paranthropus: Puzzle of a Plio-Pleistocene Hominin
When Paranthropus robustus was first described in 1938 by paleontologist Robert Broom, it was immediately clear that this creature didn’t fit neatly into any category. With a robust jaw, massive cheek teeth, and a cranium built to withstand intense chewing forces, P. robustus was a specialist—adapted to a diet that may have included tough roots, seeds, and fibrous plants. It stood short and stocky, yet its skull bore striking crests and heavy brow ridges that suggested power.
But beyond morphology, Paranthropus has remained a mystery. Where does it fit in our evolutionary story? Is it a rugged cousin of Australopithecus africanus, the so-called “southern ape of Africa”? Or is it a wholly separate lineage that branched off and ultimately died out without contributing directly to modern humans? For decades, such questions have hinged on fragmentary fossils and anatomical speculation.
Now, researchers are bringing molecular tools to bear on the mystery. Because DNA degrades rapidly in warm climates, Africa’s ancient hominins have often been out of molecular reach. Traditional genomics—so effective in cooler Eurasian contexts—has had little success south of the Sahara. But proteins, it turns out, are more resilient.
Why Tooth Enamel Holds the Molecular Key
Unlike bones, which are porous and easily infiltrated by groundwater and microbes, tooth enamel is the hardest, most mineralized substance in the human body. Its crystalline structure shields internal proteins from environmental decay, allowing for their preservation over geological timescales. This makes enamel proteins ideal targets for paleoproteomics, a rapidly advancing field that uses protein sequences to explore ancient biology.
The international team behind the Science study employed ultra-sensitive mass spectrometry techniques to extract and sequence proteins from the fossilized enamel of four P. robustus individuals unearthed from Swartkrans—a site famous for its rich hominin record. Each of these individuals lived nearly two million years ago, long before the age of Neanderthals or Homo sapiens, and well before DNA could be expected to survive.
What they found in the protein sequences was nothing short of astonishing.
Sexing the Ancient Dead: Beyond Bones and Guesses
For years, anthropologists have debated how to determine the sex of ancient hominin fossils. In species like Paranthropus robustus, which exhibits significant sexual dimorphism—where males and females differ dramatically in size and form—it was often assumed that bigger meant male, and smaller meant female. But this assumption, based primarily on size, is now on shaky ground.
The protein analysis revealed that two of the individuals were biologically female, and two were male. Yet intriguingly, the differences in their skeletal features did not always align with previous expectations based on size alone. This challenges long-standing anthropological models and suggests that skeletal size may not be a reliable indicator of sex in early hominins—at least not without molecular confirmation.
This realization opens a new window into the social lives of ancient hominins. Were females sometimes as robust as males? Could what we’ve interpreted as “male dominance” in fossil morphology be a case of mistaken identity? These are the kinds of questions that only protein-based sexing can now begin to answer.
Genetic Variation in a Stone Age Neighborhood
Beyond determining sex, the enamel proteins also carried genetic signals that allowed the team to assess variation between individuals. What they found suggests that P. robustus was more genetically diverse than previously thought.
One individual stood out as especially distinct—differing at a molecular level from the others in ways that hint at either significant intraspecies variation or possible interbreeding between groups or subspecies. It’s a tantalizing hint that P. robustus may have lived not as a single, homogenous population, but as multiple genetically distinct groups, possibly adapted to different ecological niches or social structures.
This insight aligns with emerging views in paleoanthropology that ancient hominins were more socially and genetically complex than previously assumed. Just as early Homo species likely interacted, interbred, and competed, so too might the robust australopiths have been navigating a biologically diverse world of kin and competitors.
Rethinking Evolutionary Trees: Blurred Lines and Branches
The implications of this study go far beyond P. robustus. For decades, evolutionary relationships between early hominins have been depicted as a branching tree—with clear distinctions between Australopithecus, Paranthropus, and Homo. But this model is under increasing strain.
The molecular diversity seen in these four P. robustus individuals, combined with mounting fossil evidence of overlapping traits among species, suggests that our ancestors may not have evolved in tidy branches, but in braided streams of genetic and ecological exchange. It’s possible that what we call P. robustus was not a monolithic species, but a mosaic—an umbrella term for a set of populations sharing broad features but varying locally in behavior, diet, and genes.
In this view, evolution is less like a ladder and more like a tangled river delta—full of forking paths, dead ends, and merging currents. This may help explain why the lines between Australopithecus africanus, Paranthropus robustus, and early Homo remain so difficult to draw. Nature, it seems, doesn’t respect our tidy categories.
What Protein Sequences Can Tell Us About Human Origins
As enamel proteomics matures, its potential to transform human origins research is profound. We are only beginning to understand what ancient proteins can tell us—not only about identity and sex, but also about metabolism, development, immunity, and even diet. Already, protein analysis has illuminated the ancestry of Denisovans, an archaic hominin lineage known from little more than a pinky bone. Now, it’s giving voice to even older hominins from deep in the African past.
In this case, the protein sequences don’t just tell us who these individuals were—they hint at how they lived, how they may have migrated, and how they may have mated across group boundaries. It brings us closer than ever to reconstructing the social worlds of species that left no written records, only the durable architecture of their teeth.
Looking Forward: A New Age of Molecular Paleontology
The success of this study has already sparked excitement across the field. If enamel proteins can survive for two million years in the hot, dry caves of South Africa, then similar techniques may soon be applied to other early hominin species—Australopithecus afarensis, Homo habilis, even the famously elusive Homo naledi. With every new sequence, we step closer to a time machine made not of gears and lights, but of peptides and amino acids.
Importantly, this also underscores the necessity of ethical, careful sampling. Fossil teeth are finite, irreplaceable treasures. As molecular methods become more precise, researchers are working to balance the destructive nature of sampling with the incredible insights it can unlock.
Conclusion: The Voices in the Enamel
At first glance, a fossilized tooth is just a remnant—a calcium artifact in stone. But now, we know better. Locked within that enamel is a molecular memoir, one that tells us who these ancient beings were, how they lived, and how they were connected to one another.
The four individuals from Swartkrans were not abstractions. They were real, living hominins—male and female, similar and different, possibly even strangers who met across the Stone Age savannah. Their teeth, long buried, are now singing into the present, expanding our vision of what it meant to be human in a time before history.
Thanks to protein science, Paranthropus robustus is no longer silent. Its enamel, against all odds, has endured. And with it, a deeper, more intimate story of our evolutionary cousins is being told—one tooth at a time.
Reference: Palesa P. Madupe et al, Enamel proteins reveal biological sex and genetic variability in southern African Paranthropus, Science (2025). DOI: 10.1126/science.adt9539