Every moment, billions of cells in the human body quietly die and are replaced by new ones. This constant renewal is essential for life—clearing away damaged cells, shaping growing tissues, and keeping inflammation at bay. But what if this vital process also holds a hidden vulnerability, one that viruses could exploit to spread undetected?
In a groundbreaking study from La Trobe University in Australia, scientists have uncovered a previously unknown way viruses might travel through the body. Published in Nature Communications, the discovery sheds light on an overlooked phase of cell death—one that could change how we understand infection, immunity, and the future of medicine.
The Mystery of Dying Cells
When a cell reaches the end of its life, it doesn’t simply vanish. Instead, it undergoes a carefully orchestrated process known as apoptosis, or programmed cell death. This is nature’s way of recycling: the cell dismantles itself piece by piece so the immune system can safely clean up the remains.
Until now, researchers believed this process was fairly straightforward—cells break apart, and their fragments are swiftly removed. But the team at La Trobe University, led by Ph.D. candidate Stephanie Rutter in Professor Ivan Poon’s lab at the La Trobe Institute for Molecular Science (LIMS), discovered that this final act of cellular self-destruction is far more complex and deliberate than anyone realized.
The Discovery of a Hidden Signal: The “Footprint of Death”
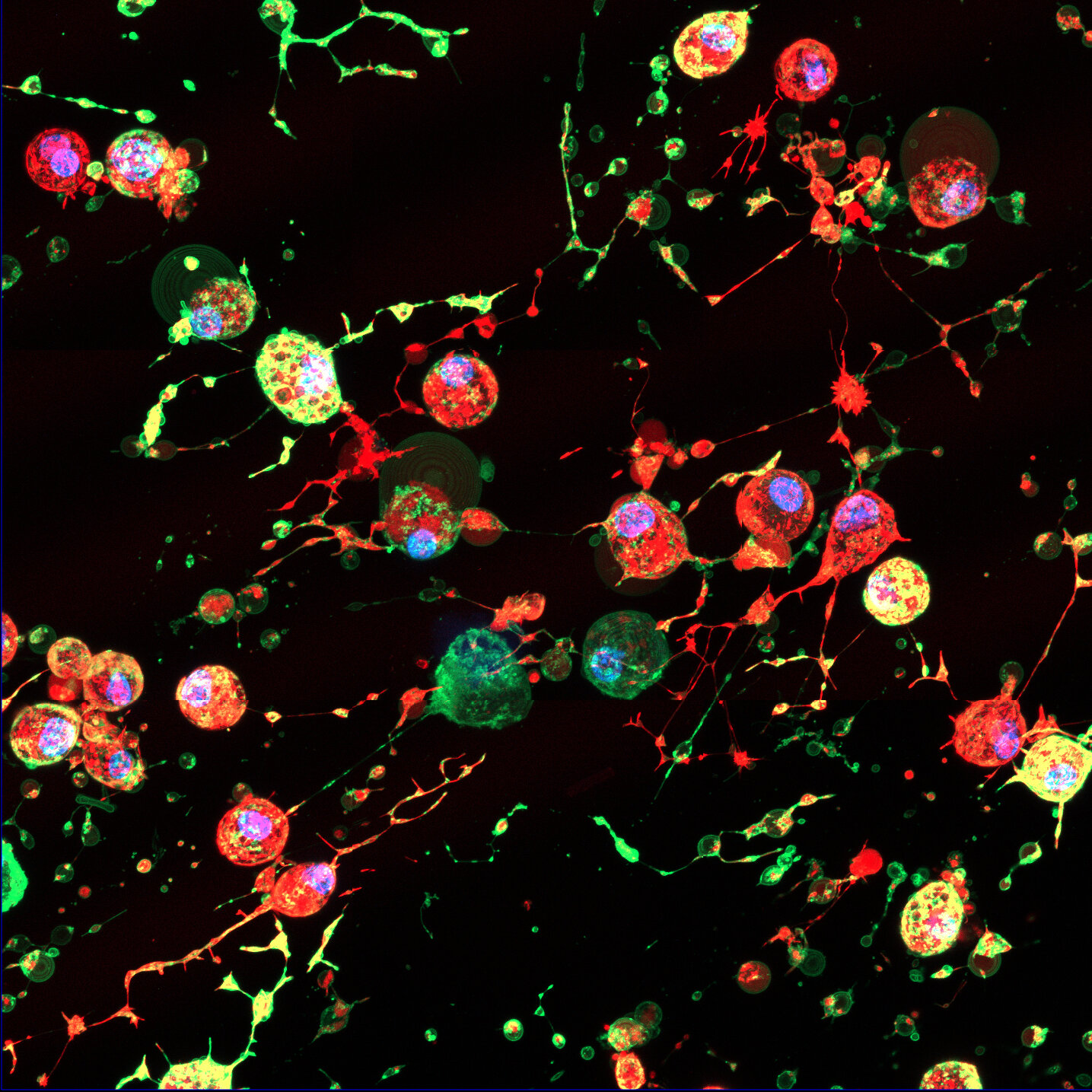
As dying cells begin to self-destruct, they change shape and physically lift away from their surroundings. What they leave behind is a kind of biological residue—an invisible marker scientists have dubbed the footprint of death.
Within this footprint, researchers found something completely new: a distinct type of extracellular vesicle (EV)—tiny, membrane-bound packages that cells use to send signals and materials to one another. These newly discovered vesicles, called F-ApoEVs, appear to act as biological breadcrumbs, helping the immune system find and clean up the remains of dead cells before they cause inflammation or damage.
Extracellular vesicles are well-known messengers of the body, carrying proteins, lipids, and genetic material between cells. But the identification of F-ApoEVs adds a surprising new layer to this communication network—one that only emerges when cells die.
When Viruses Turn the Body’s Cleanup Crew into an Accomplice
What makes this discovery especially remarkable—and concerning—is what happens when viruses enter the picture. The research team observed that when cells infected with influenza undergo apoptosis, the virus can hijack the cleanup process.
Instead of being destroyed, viral particles hide inside the newly formed F-ApoEVs. These tiny vesicles, which normally help the immune system clear debris, can instead serve as covert vehicles, carrying the virus to nearby healthy cells.
This finding suggests that viruses might exploit the body’s natural mechanisms of cell clearance to silently spread infection. It’s a stunning revelation that redefines what we know about viral behavior inside the body.
A Window into the Complexity of Life and Death
Professor Ivan Poon, Director of the Research Center for Extracellular Vesicles (RCEV) at La Trobe, believes this research could transform how scientists think about disease at a cellular level.
“Understanding this basic biological process could open new avenues of research to develop treatments that help the immune system better fight disease,” he said. “Billions of cells are programmed to die each day, and we used to think this process was random and simple. Our findings show it’s actually a finely tuned sequence—each step is critical to ensure dying cells are broken down and cleared efficiently.”
In other words, cell death isn’t just an end—it’s a carefully choreographed transition that maintains the delicate balance of life. And now, we know it’s a process that can also be manipulated by pathogens.
The Role of Communication in Cellular Health
Lead researcher Stephanie Rutter emphasizes that the study highlights just how essential cell-to-cell communication is to maintaining health. “The body needs to clear away dead cell fragments to prevent them from lingering and causing inflammation or autoimmune diseases such as lupus,” she explained. “We saw that F-ApoEVs are readily cleared from the site of cell death. But what we didn’t expect was how viruses can take advantage of this process.”
By hiding inside F-ApoEVs, viruses essentially gain a free pass through the body’s natural defense systems—blending in with the harmless debris of normal cell turnover. This discovery doesn’t just deepen our understanding of how infections spread; it could also reveal why certain viruses are so hard to eradicate.
From Death Comes Discovery
The research also underscores the poetic paradox at the heart of biology: that death and life are inextricably linked. Dying cells don’t simply vanish—they continue to “speak” to their environment, guiding immune responses and influencing neighboring cells even after their demise.
Dr. Georgia Atkin-Smith from the Walter and Eliza Hall Institute (WEHI), a co-leader of the study, put it succinctly: “This study has revealed that dying cells can continue to communicate from the grave and may impact immune function.”
This lingering communication is both a marvel and a potential threat. It’s a reminder that even the most well-intentioned biological processes can be exploited by nature’s most cunning invaders.
A New Frontier for Drug Development
The implications of this discovery are enormous. If viruses can use F-ApoEVs as vehicles of infection, then targeting these vesicles—or the processes that produce them—could become a powerful strategy for future therapies.
By better understanding how the immune system identifies and removes dying cells, researchers might one day design drugs that prevent viruses from hijacking this mechanism. Similarly, manipulating F-ApoEVs could offer new ways to control inflammation, treat autoimmune diseases, or even improve cancer immunotherapies.
Professor Poon believes this marks the beginning of a new era in biomedical research: “The more we understand about cell death and what happens afterward, the better equipped we are to understand diseases and find new treatments.”
The Broader Meaning of the Discovery
At its core, this study reveals a profound truth about biology: every process, no matter how routine, holds unseen depth and complexity. For decades, scientists thought they had mapped the major stages of cell death. But now, this discovery adds a hidden chapter—one that bridges death, communication, and infection in an elegant and unexpected way.
It’s a reminder that even after centuries of study, the body still holds secrets. The microscopic dance of dying cells and their molecular messages continues to surprise us, showing that nature’s designs are not only intricate but purposeful.
Looking Ahead
The La Trobe team’s work represents only the beginning. Future studies will explore how widespread this viral hijacking mechanism might be across different pathogens, and whether similar vesicles play roles in other diseases. If influenza can exploit F-ApoEVs, could other viruses—perhaps even coronaviruses—do the same?
Answering these questions could reshape how we approach infectious disease treatment. The discovery also raises exciting possibilities for regenerative medicine and immunology, where understanding the dialogue between dying and living cells could lead to breakthroughs in healing and recovery.
The Silent Language of Life and Death
Perhaps the most striking lesson from this research is that death, at least in biological terms, is not silence—it’s communication. Dying cells leave behind signals that guide the immune system, maintain balance, and preserve health. In this sense, death itself becomes part of life’s ongoing conversation.
But when viruses learn to speak that same language, the conversation turns dangerous. By mimicking or hiding within the body’s messages, they blur the line between self and invader, protection and infection.
More information: Stephanie F. Rutter et al, The formation of the ‘footprint of death’ as a mechanism for generating large substrate-bound extracellular vesicles that mark the site of cell death, Nature Communications (2025). DOI: 10.1038/s41467-025-64206-3