In the realm of cancer biology, uncovering where a tumor truly begins—its “cell of origin”—has long been a challenge. Especially in lung cancer, the leading cause of cancer deaths worldwide, the origins have remained elusive. But now, a groundbreaking study by researchers at UCL, the Wellcome Sanger Institute, and the University of Cambridge has shed light on a pivotal discovery: the second most common form of lung cancer, lung squamous cell carcinoma (LUSC), originates from a very specific group of cells in the trachea. These basal cells, identified by their expression of a gene called Krt5, don’t just mutate—they evolve into conquerors, expanding across the lung in a slow but relentless invasion that can eventually lead to deadly tumors.
This revolutionary research, published in the journal Science, is more than just a molecular mystery unraveled. It opens a window into the earliest changes in the lung that might be detected and potentially halted before cancer has a chance to bloom.
An Unexpected Battlefield: The Trachea as Ground Zero
The story of lung squamous cell carcinoma begins far from the typical site of a tumor. It begins in the trachea—the windpipe—home to a layered architecture of cells with distinct roles. Among them are basal cells, quiet architects that help maintain the airway’s structure and repair damage. Under normal circumstances, these cells are not aggressive; they divide modestly and differentiate into more specialized cells such as ciliated or secretory luminal cells, which are essential for trapping and clearing debris from the lungs.
However, when exposed to persistent damage, particularly from carcinogens like tobacco smoke, these basal cells change. They begin to outcompete their neighbors, altering the cellular ecosystem of the airway. This transformation is not random. It is orchestrated and progressive—an evolutionary conquest played out cell by cell, gene by gene.
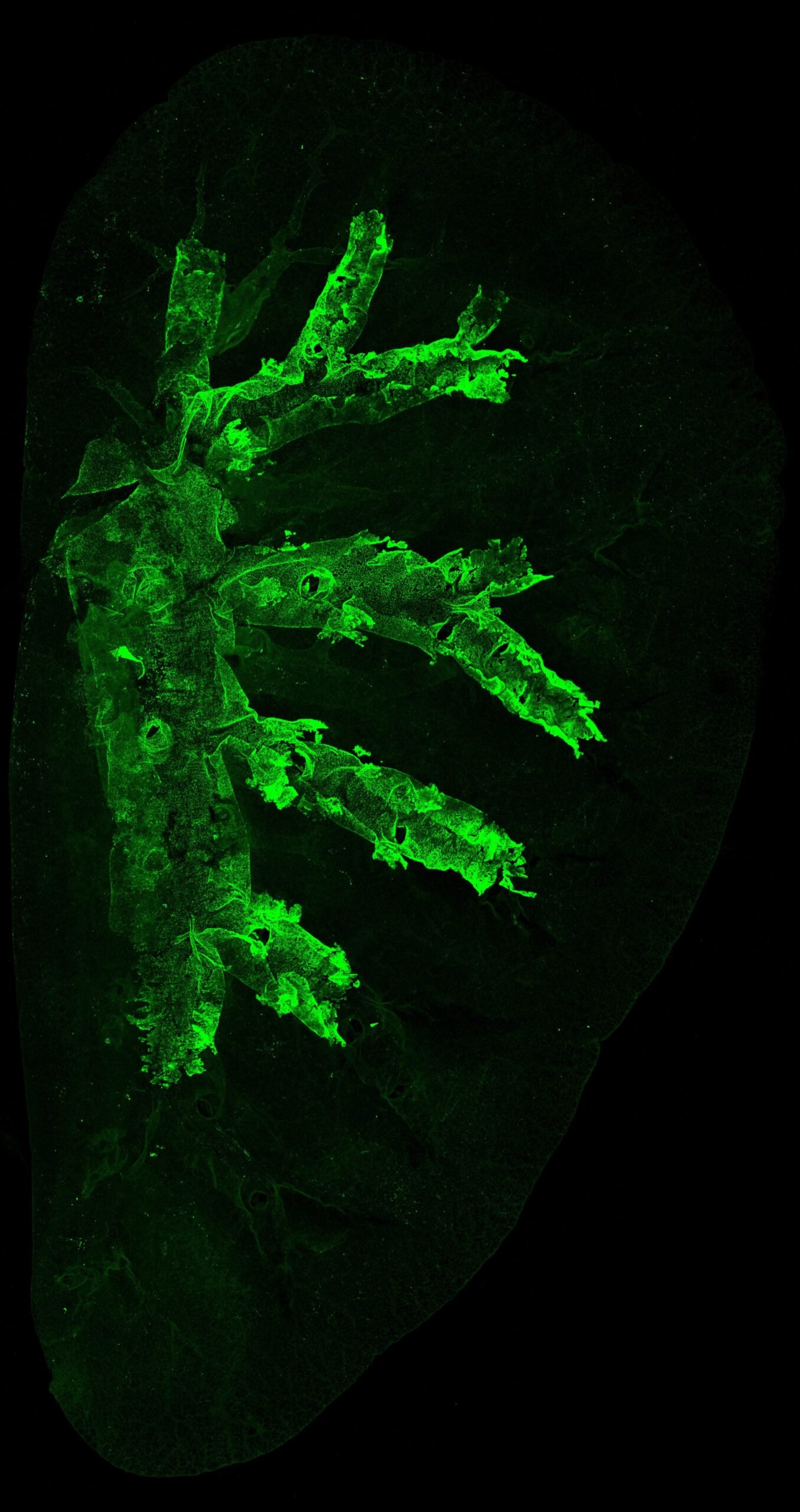
At the heart of this transformation is Krt5, a gene crucial to cellular scaffolding. When researchers labeled Krt5-expressing cells in mice, they were able to track the journey of these basal cells over time. In mice exposed to carcinogens, these cells multiplied and began to spread from their home in the trachea into the delicate tissue of the lung itself. This colonization was the precursor to cancer.
The Great Cellular Takeover
In the context of cancer, what begins as a subtle shift in cellular behavior can eventually cascade into systemic dominance. The research team discovered that once a few damaged basal cells began to proliferate, their descendants rapidly outcompeted normal cell populations. The normal balance between basal and luminal cells was lost. Like an invasive species, these Krt5 cells spread—first across the trachea, then deeper into the bronchial branches, and ultimately across whole lung lobes.
This expansion was dramatic. In many cases, entire sections of lung tissue became populated by the descendants of just a few aberrant basal cells. It is from these cellular dynasties that lung squamous cell carcinomas arise.
This finding changes the narrative of cancer development. Rather than a single cell turning malignant in isolation, this is a story of cellular dominance—of environmental conquest. The cancer is not just born; it is built, layer upon layer, as the invading cell population conquers new terrain.
A War Waged at the Genetic Level
To understand why these cells were able to expand so aggressively, the scientists dug deeper into their molecular biology using single-cell RNA sequencing. By comparing gene expression patterns between healthy and carcinogen-exposed mice—as well as human airway cells from smokers and non-smokers—they discovered that damaged basal cells weren’t merely reproducing. They were transforming.
A specific transitional cell type, expressing the gene Krt13, emerged during this process. These transitional cells were more abundant in damaged airways and signaled a state of instability—a kind of halfway house between normal and malignant. At the same time, critical luminal cells were disappearing, reducing the lung’s ability to defend and repair itself.
The findings were mirrored in humans. DNA sequencing of airway tissues from former smokers revealed that separate precancerous lesions across different lung areas were all derived from the same clonal population of basal cells—confirming the theory of a single-cell origin and clonal expansion across multiple regions.
In this sense, the lung is not just a passive field where cancer sprouts randomly. It is an active battleground, and the outcome is decided long before visible tumors arise.
Why This Discovery Matters: Toward Early Detection and Prevention
Cancer is most treatable when caught early—but lung cancer is rarely detected at a curable stage. That’s largely because early cellular changes are subtle and invisible to conventional imaging. This study provides the first blueprint of those early steps. By identifying the dominant clonal expansion of basal cells as a precursor to cancer, researchers may be able to develop biomarkers—molecular fingerprints—to detect lung cancer while the tissue still appears healthy.
Even more tantalizing is the possibility of prevention. If scientists can understand why and how Krt5-expressing cells become dominant—why they are “selected” for survival and expansion—they may be able to develop drugs that stop these cells in their tracks.
Such drugs wouldn’t need to target tumors. They could be administered during a precancerous phase, reducing the chances of cancer forming at all.
The Ecological View of Cancer
Professor Peter Campbell, co-author of the study, emphasized an often-overlooked truth: cancer is not just a mutation-driven disease. It is ecological. Cells respond to their environment, and in turn, shape it.
“What we found,” he said, “was that squamous cell lung cancer doesn’t arise until a damaged cell finds the right environment—an ecological niche in the lung that gives it space to grow and mutate further.”
This “niche” could be an area of the lung where normal cells have died off due to damage, or where inflammation has altered the local biology. Once the right conditions arise, the damaged basal cells seize the opportunity.
It’s a sobering realization: mutations alone aren’t enough. Cancer requires not just a bad actor, but a bad stage.
Implications for Future Research and Treatment
This research represents a paradigm shift in our understanding of lung cancer. It illustrates how cancerous transformation is not just a genetic event but also a spatial and ecological one. Just as invasive plant species need the right soil to flourish, these aggressive basal cells need the right environment to dominate.
Armed with this knowledge, researchers are now asking deeper questions. Can we reverse the conditions that allow these cells to spread? Can we restore the balance of cell populations in the lung after exposure to carcinogens? Could drugs that inhibit the signaling pathways of Krt5-expressing cells slow or even halt their expansion?
These questions are no longer theoretical—they are within reach.
From the Lab to the Clinic: Bridging Science and Survival
Dr. Talisia Quallo of Cancer Research UK highlighted the practical implications of the study. “This research reveals the early cellular steps preceding the development of lung squamous cell carcinoma,” she said. “More research is needed, but in the future this information could help scientists develop new ways to detect the disease earlier or even prevent it from occurring.”
Indeed, this study is not just a triumph of cell biology—it’s a potential bridge between the laboratory and the clinic. One day, a patient could undergo a simple test that screens for early signs of basal cell dominance in their lungs, leading to an early intervention and potentially a lifesaving outcome.
Conclusion: A New Chapter in the War Against Lung Cancer
Lung squamous cell carcinoma, long a mysterious and lethal foe, has now been traced to its origin. It is not a chaotic disease, but a structured and predictable one—an orchestrated expansion of damaged basal cells that hijack the lung’s architecture.
By identifying the early events in this process, this study has opened a powerful new front in the fight against lung cancer. What was once invisible is now visible. What was once inevitable may soon be preventable.
The war against lung cancer is far from over, but we now know where the first shots are fired. And that knowledge may ultimately change everything.
Reference: Sandra Gómez-López et al, Aberrant basal cell clonal dynamics shape early lung carcinogenesis, Science (2025). DOI: 10.1126/science.ads9145. www.science.org/doi/10.1126/science.ads9145