In a groundbreaking evolution of diabetes therapy, scientists have made a major leap toward a sustainable, potentially curative treatment for type 1 diabetes. For decades, the primary strategy for treating this autoimmune disease has revolved around the administration of exogenous insulin. But now, the frontier of regenerative medicine has begun to unlock something far more transformative: the ability to generate insulin-producing pancreatic islets from stem cells—on demand, in labs, and at a scale that could reach every patient in need.
This revolutionary approach not only addresses the scarcity of donor tissue, which has long limited the widespread use of islet transplantation, but also holds the promise of fundamentally changing how type 1 diabetes is managed, possibly eliminating the need for daily insulin injections for many. At the heart of this innovation is a blend of sophisticated cell biology, regenerative engineering, and clinical insight—all converging to offer new hope where before there was only maintenance.
The Long Quest for Islet Cell Transplants
The dream of replacing lost islet cells in people with type 1 diabetes is not new. It began over fifty years ago, when researchers attempted to isolate pancreatic islets from deceased donors for transplantation. These tiny clusters of cells—specifically the insulin-producing beta cells—are crucial for regulating blood sugar levels. However, this method faced serious limitations: a shortage of donor pancreases, high rates of cell death during extraction and transplantation, and the risk of immune rejection.
Despite these hurdles, the procedure matured over time. By the early 2000s, islet transplantation emerged as a viable treatment for certain individuals, particularly those with life-threatening hypoglycemic unawareness. In Canada and parts of Europe, this approach is now approved, though it remains investigational in the United States. Still, the therapy was hamstrung by one critical bottleneck—supply.
Pancreatic islets from deceased donors are limited in number and often incompatible due to immune mismatches. Moreover, most recipients still need to take immunosuppressive drugs to prevent rejection, and many continue to require insulin, albeit in smaller doses.
This brought researchers to a bold question: could we engineer islet cells ourselves?
Engineering Beta Cells from Scratch
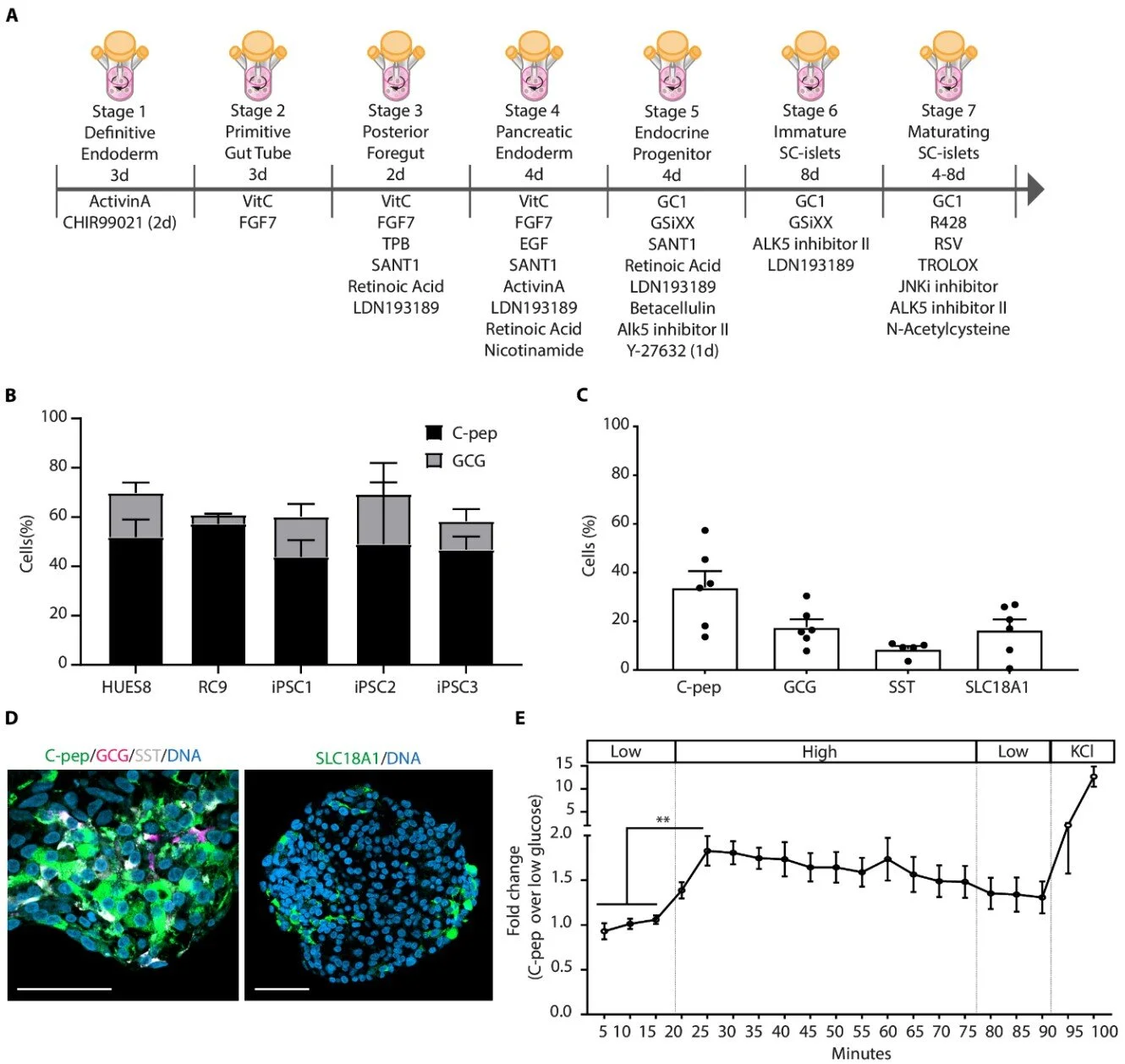
Enter stem cell technology. In a series of dazzling advances, researchers, including a leading team in the Netherlands, have taken pluripotent stem cells—cells capable of becoming any type of cell in the body—and coaxed them into forming functional beta islets. These stem cell-derived islets are biologically similar to their naturally occurring counterparts and possess the vital ability to sense glucose levels and secrete insulin accordingly.
Led by Dr. Bahareh Rajaei of Leiden University Medical Center, the Dutch team recently published landmark findings in Science Translational Medicine, demonstrating that their lab-grown islets not only produce insulin but maintain their structure and functionality when transplanted into animal models.
“The progressive loss of insulin-producing β cells is a hallmark of type 1 diabetes,” Rajaei writes, underlining the condition’s origin in autoimmunity, where the body’s own defenses destroy the beta cells. “An insufficient functional β cell mass leads to hyperglycemia—which, if left untreated, can be fatal.”
Rajaei’s team has done more than just generate these vital cells; they’ve refined how to do it with unprecedented precision.
Preserving the Architecture of Life-Saving Cells
One of the challenges in creating transplant-ready islets has been preserving their internal “architecture”—the delicate structural integrity that enables beta cells to communicate and function properly. Earlier techniques often led to cells clumping together incorrectly or failing to fully develop. Worse, purification steps sometimes damaged the cells, rendering them ineffective.
But the Dutch researchers have engineered a purification process that safeguards this architecture. By using induced pluripotent stem cells (iPSCs)—reprogrammed adult cells that revert to an embryonic-like state—they were able to guide the cells through a multistep differentiation protocol. The end result: islets that are not only functional but structurally viable for transplantation.
What makes this technique even more significant is its scalability. These stem cell-derived islets can be produced in large quantities, on demand, and using clinical-grade manufacturing protocols. This could obliterate the donor shortage problem and open the door to widespread therapeutic application.
Global Implications for Type 1 Diabetes
Type 1 diabetes is not a niche disease. According to the World Health Organization, more than 8.75 million people worldwide were living with the condition as of 2022. Many are diagnosed in childhood or early adulthood, facing a lifetime of glucose monitoring, dietary vigilance, and insulin injections.
For these individuals, the possibility of replacing damaged beta cells through a single or periodic infusion of stem cell-derived islets could mean freedom—not just from needles and pumps, but from the looming threat of complications such as blindness, kidney failure, or neuropathy.
And while the journey is not over, clinical trials are already bringing this futuristic vision closer to reality. In the United States, companies like Vertex Pharmaceuticals have shown that stem cell-derived islets can survive and function after transplantation into humans. These trials, while still early-stage, hint at the transformative potential of regenerative medicine.
Beyond Insulin: A Cellular Revolution
Rajaei’s work is part of a broader shift in medicine: treating diseases at the cellular level. Rather than merely managing symptoms with drugs, the idea is to repair or replace the damaged tissues and cells themselves. This approach is gaining traction in areas as diverse as neurodegeneration, cardiac disease, and hematological disorders.
The techniques pioneered for beta cell development—precise differentiation, structural preservation, and immune evasion—could be adapted for other regenerative therapies. Whether it’s neurons for Parkinson’s disease, cardiomyocytes for heart failure, or hematopoietic cells for blood disorders, the possibilities are expanding rapidly.
In the case of diabetes, the road ahead still includes some bumps. Immune rejection remains a concern, especially since type 1 diabetes is an autoimmune disorder. Even if the beta cells are lab-made, the immune system might still attack them unless protective strategies—like encapsulation or gene editing—are employed.
Moreover, long-term studies are needed to determine how long these transplanted cells survive and how reliably they perform under the body’s dynamic conditions.
A New Chapter in Diabetes Care
Still, the writing is on the wall. We are entering a new era in diabetes care—one in which regenerative medicine may move us from lifetime management to potential remission or cure. The advances in stem cell biology, spearheaded by Rajaei’s team and others around the world, are turning decades of research into actionable therapies.
Their findings have drawn the attention not just of endocrinologists and scientists, but also of patients and families yearning for a future less burdened by needles, monitors, and fear.
As Rajaei concluded in her study, “This method may also contribute to the generation of improved cell-based therapies for regenerative medicine purposes beyond the stem cell-islet field.” In other words, this isn’t just a victory for diabetes. It’s a proof of concept for an entirely new medical paradigm.
One in which cells—not drugs—become the frontline treatment.
One in which the body, with the help of science, can begin to heal itself.
And one that may, at long last, deliver a long-awaited promise to millions: a life without diabetes.
Reference: Bahareh Rajaei et al, Clinically compliant enrichment of human pluripotent stem cell–derived islets, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adl4390