When the world learned that Betsy Arakawa—the classical pianist and wife of the late actor Gene Hackman—had died from a hantavirus infection, many people were hearing the name of the virus for the very first time. Yet for decades, scientists have known that hantaviruses, though relatively rare, are among the most dangerous pathogens transmitted from animals to humans. With mortality rates ranging anywhere from 1% to 40%, depending on the strain, they command both respect and fear in the medical community.
Now, researchers at UCLA have taken a groundbreaking step toward understanding how these elusive viruses attack the human body. By growing miniature organ-like structures—organoids—made from human stem cells, they are revealing in unprecedented detail how hantaviruses spread, damage tissues, and, importantly, how they might be stopped.
What Makes Hantaviruses So Dangerous?
Hantaviruses are carried by rodents and can spread to humans when people breathe in dust contaminated by rodent droppings, urine, or saliva. Once inside the body, they can cause two serious diseases: hantavirus pulmonary syndrome (HPS), marked by catastrophic lung failure, and hemorrhagic fever with renal syndrome (HFRS), which damages the kidneys.
In the United States, the Sin Nombre virus has been responsible for deadly outbreaks of HPS, particularly in the Southwest. Across Asia, the Hantaan virus causes severe kidney disease. But the strain most concerning to scientists today is the Andes virus, found in South America, which is the only hantavirus known to spread from person to person.
The Andes virus poses an especially urgent threat: recent deaths in California and New Mexico remind us that global travel and ecological changes can extend its reach beyond traditional boundaries.
The Promise of Organoids
Studying hantaviruses in the lab has always been fraught with challenges. The viruses require biosafety level 4 containment—the highest level of biocontainment reserved for the most dangerous pathogens. Traditionally, researchers have relied on hamsters to model infection, but such animal models fail to capture the complexity of human tissues.
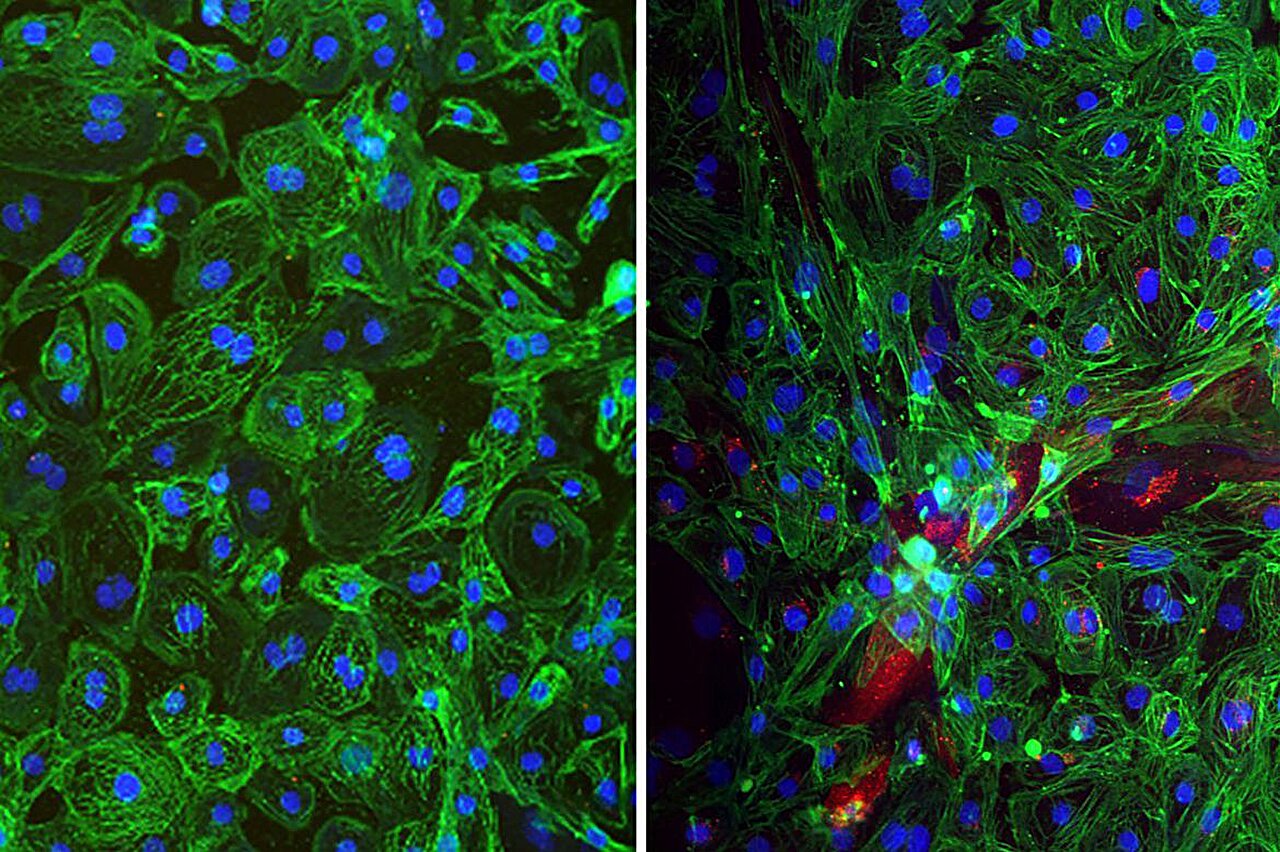
Enter organoids: three-dimensional, stem-cell-derived structures that mimic the architecture and function of real human organs. These “mini-organs” allow scientists to model infection in lung, heart, and brain tissues without putting human patients at risk.
“This is the first time we’ve been able to watch hantaviruses infect human organ systems directly,” said Dr. Vaithi Arumugaswami, senior author of the new study. “It opens the door to a whole new way of studying these deadly pathogens.”
How Hantaviruses Invade the Body
The UCLA team exposed organoid models of lungs, hearts, and brains to three major hantaviruses—Andes, Hantaan, and Sin Nombre—and the results were striking.
The Andes virus proved alarmingly versatile. It infected every type of cell tested: the epithelial and endothelial cells of the lungs, the cardiomyocytes of the heart, and even the astrocytes of the brain. By contrast, the Hantaan virus showed a more selective pattern, infecting mainly heart and brain cells, while the Sin Nombre virus largely restricted itself to lung cells.
“This is the first evidence that Andes virus can replicate efficiently in human lung organoids,” noted Arunachalam Ramaiah, a co-senior author of the study. “It shows just how adaptable—and dangerous—this virus is.”
Even more troubling, the Andes virus didn’t simply invade cells; it rewired them. In lung tissue, it sparked inflammation and injury while shutting down key metabolic pathways involved in processing cholesterol and fats. In the heart, it disrupted the cell’s structure so severely that the miniature heart organoids stopped beating rhythmically.
“The virus reprograms cell metabolism to favor its own survival,” explained Arumugaswami. “That disruption may explain the devastating lung and heart damage we see in patients.”
Searching for a Cure
Currently, there are no approved treatments or widely available vaccines for hantavirus infections. Supportive care—such as oxygen therapy for respiratory distress—remains the only option for patients.
The UCLA team, however, identified two compounds that showed significant promise. The first, urolithin B, is a natural molecule found in certain fruits and nuts. It not only blocked Andes virus infection in the lab but also restored normal metabolism in infected cells, all while leaving healthy cells untouched. The second, favipiravir, is an antiviral drug already approved in Japan for treating influenza. It too blocked viral replication in organoid models.
“Two compounds, urolithin B and favipiravir, showed real promise,” said Nikhil Chakravarty, a medical student and co-author of the study. “It’s early work, but it points us toward potential therapies where none currently exist.”
The Role of Climate Change
Though hantavirus infections are still relatively rare in the United States, the risk of exposure is growing. As climate change alters ecosystems, rodent habitats are shifting. Warmer temperatures, combined with urban expansion, may drive rodents closer to human dwellings, increasing the likelihood of viral transmission.
“The impacts of climate change may bring people into more frequent contact with hantavirus carriers,” explained Arjit Vijey Jeyachandran, the study’s first author. “That makes it even more important to understand how these viruses spread—and how we can stop them.”
A New Frontier in Virology
The UCLA study, published in PLOS Pathogens, represents a major step forward in the battle against hantaviruses. By creating organoid models of the human lung, heart, and brain, researchers can now explore how these viruses exploit the body at a level of detail previously impossible. Just as importantly, the system provides a testing ground for potential antiviral therapies—an urgently needed tool in the absence of vaccines or approved treatments.
Hantaviruses, once obscure, are no longer viruses we can afford to ignore. Their deadly potential, coupled with environmental shifts that may bring them into closer contact with humans, makes them an urgent public health concern. But with new tools like organoids, scientists are better equipped than ever to unravel their mysteries.
The story of hantaviruses is one of danger, but also of scientific ingenuity. Out of tragedy comes determination, and out of curiosity comes hope. If organoid models can help unlock the secrets of how hantaviruses kill, they may also be the key to saving lives.
More information: Arjit Vijey Jeyachandran et al, Differential tropisms of old and new world hantaviruses influence virulence and developing host-directed antiviral candidates, PLOS Pathogens (2025). DOI: 10.1371/journal.ppat.1013401