After decades of dashed hopes and biological roadblocks, scientists may finally be closing in on a long-sought goal: an effective HIV vaccine. Two new studies published in Science Translational Medicine signal a major advance, showing that messenger RNA (mRNA)—the same technology used in COVID-19 vaccines—can be engineered to focus the immune system’s firepower more precisely on the parts of HIV that matter most. It’s a development that, while not yet a breakthrough, is a meaningful stride in one of the most complex challenges modern medicine has ever faced.
The enemy is as cunning as it is deadly. HIV, the virus that causes AIDS, mutates rapidly and wears a molecular disguise to evade the immune system. Most vaccines have struggled to provoke the kind of neutralizing antibodies needed to disarm the virus. But these new mRNA-based approaches are showing that with the right instructions, our cells might just learn to produce defenses that go for HIV’s jugular rather than its smoke and mirrors.
Understanding the Problem: Why HIV Has Been So Hard to Vaccinate Against
What makes HIV so uniquely difficult to target is the very thing that makes it dangerous—it’s a master of disguise. The virus hides behind a cloak of constantly shifting proteins, called the envelope (Env) glycoprotein trimer, which sits on its surface like a decoy-laden shield. Neutralizing antibodies—proteins produced by the immune system to lock onto and deactivate pathogens—have trouble identifying a consistent place to latch onto. Most vaccines, when tested, end up triggering antibodies that recognize parts of the virus that don’t actually stop it from entering cells.
So far, most experimental HIV vaccines have focused on training the immune system using soluble Env trimers—versions of the virus’s spike that float freely after being produced by a cell. These molecules mimic the structure of the real viral surface but are not physically anchored to anything. Unfortunately, while they do encourage the immune system to produce antibodies, the antibodies are often the wrong kind: nonneutralizing, and ultimately ineffective.
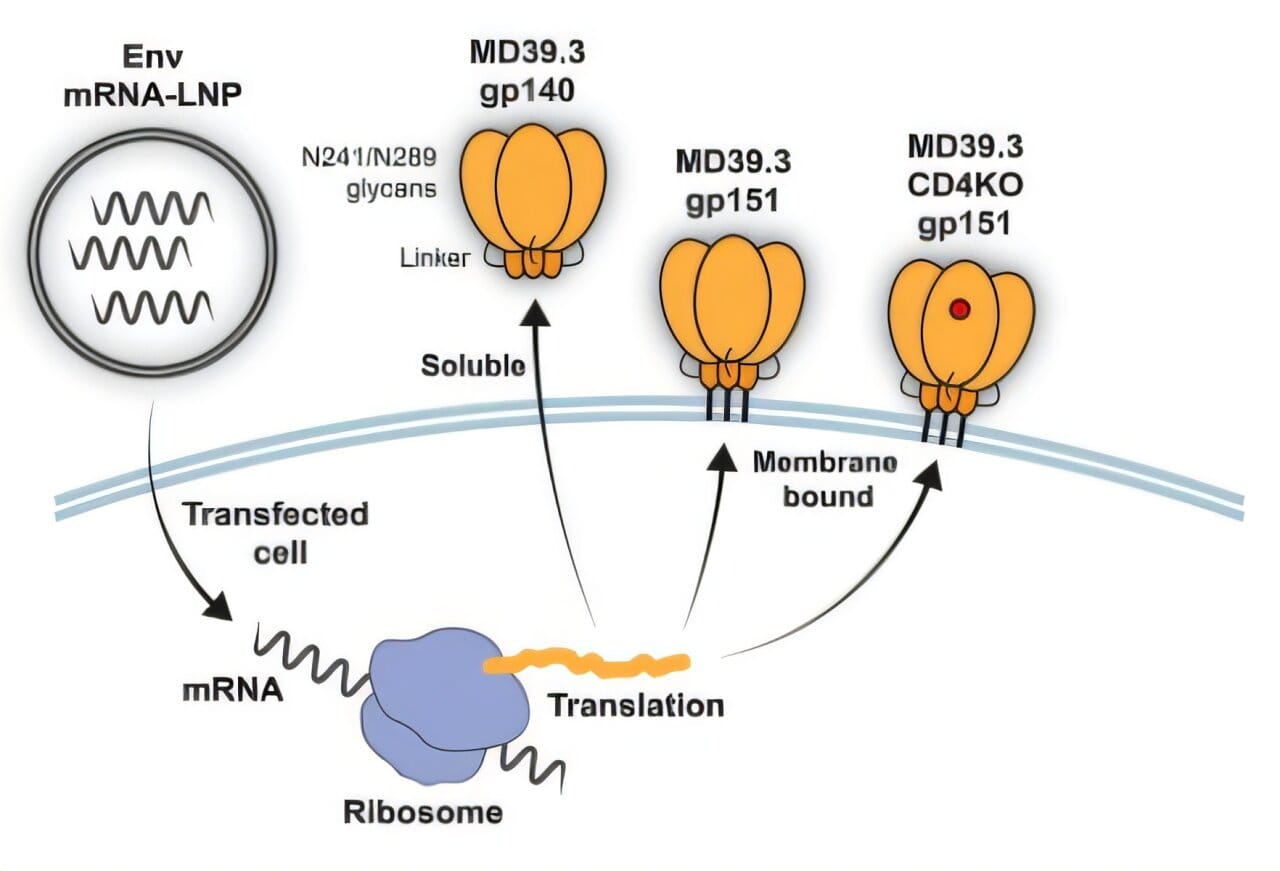
What the Scripps Research and Fred Hutchinson teams have done is pivot this strategy. Instead of relying on freely-floating viral proteins, their new vaccine designs use membrane-bound Env trimers, embedded directly into the surface of host cells, giving the immune system a more realistic—and more vulnerable—version of the virus to learn from.
The Science Behind the Innovation: Training the Immune System to Aim Better
In the first of the two studies, scientists from the Scripps Research Institute engineered a stabilized HIV Env trimer, called BG505 MD39.3, and delivered it to animals using mRNA technology. One version was soluble; the other was membrane-anchored. The results were both promising and revealing.
In rabbits and rhesus macaques, the membrane-bound mRNA vaccine consistently outperformed its soluble counterpart. Not only did it provoke higher levels of neutralizing antibodies, but it also spurred stronger CD4+ T cell activity and reduced the production of off-target memory B cells—an important marker that the immune system was learning to focus on the virus’s critical weaknesses rather than being distracted by decoys.
A year after vaccination, researchers were still detecting Env-specific plasma cells in the animals’ bone marrow—suggesting a long-lasting immune memory, the holy grail of vaccine development. This longevity, especially in the notoriously evasive context of HIV, was striking.
A Historic First: Neutralizing Responses in Humans Using mRNA Technology
The companion study, led by researchers at the Fred Hutchinson Cancer Center, took this approach a step further: into human trials. Conducted across ten sites in the United States, the phase 1 trial enrolled 108 healthy HIV-negative adults aged 18 to 55. Participants were randomly assigned to receive one of several versions of the vaccine: either soluble or membrane-bound Env trimers encoded by mRNA, or a third version with a mutation designed to reduce instability in the trimer structure.
After three immunizations, 80% of the individuals who received the membrane-bound version developed tier 2 neutralizing antibodies—a benchmark considered much closer to what would be required to block actual HIV infection. These antibodies didn’t just appear briefly after injection. They were still detectable six months later, and in many cases, had increased in strength following the third dose.
Equally important, the immune responses were well-directed. Participants produced more antibodies that targeted the “functional” parts of the HIV trimer—the very regions involved in attaching to and entering host cells. These are the virus’s most conserved regions, the ones less likely to mutate from one strain to another, and therefore the most valuable real estate for a vaccine to attack.
Some Caution Remains: Safety and Breadth Still a Challenge
No scientific progress comes without caveats. Though the immune responses were strong, they were largely strain-specific. This means that while the vaccine worked well against the particular version of HIV used in the study, it’s still uncertain how broadly protective it would be against the diverse global strains of the virus. HIV remains infamous for its genetic variability, and future vaccine candidates will need to elicit antibodies that recognize a much wider swath of viral types.
Safety, too, raised some concern. About 6.5% of vaccine recipients developed a condition called chronic urticaria—essentially persistent hives. While most cases were mild and resolved with antihistamines, a small number lingered for more than two years. One participant even required brief hospitalization. Importantly, all vaccine variants were implicated, so this side effect was not unique to the membrane-bound design. Still, it’s a signal researchers will need to monitor closely in future trials.
A Glimpse of the Future: Why This Matters
Despite the limitations, the significance of these findings cannot be overstated. For the first time, mRNA vaccines have successfully triggered neutralizing antibody responses to HIV in human participants—a benchmark many researchers feared might never be reached. The membrane-bound design, in particular, appears to give the immune system a better “look” at the virus, enabling it to prepare more effectively for the real thing.
This achievement follows on the heels of the mRNA revolution sparked by COVID-19 vaccines. But where SARS-CoV-2 was a relatively straightforward viral target compared to HIV, these studies show that the mRNA platform can be pushed further—engineered to express complex, finely-tuned antigens that do more than just provoke a response; they train precision immunity.
Researchers are already planning next steps. They hope to combine these membrane-bound mRNA vaccines with immunogens that gradually “teach” the immune system how to evolve broadly neutralizing antibodies, capable of targeting many forms of HIV. This process—known as germline targeting and affinity maturation—has been a theoretical goal for years, but these studies provide the delivery tool that may make it possible.
Toward a World Without AIDS?
HIV has killed more than 36 million people since the beginning of the epidemic and continues to infect more than a million people every year. While antiretroviral therapy has transformed HIV from a death sentence to a chronic condition for those with access, prevention remains key. A vaccine could shift the global balance, especially in parts of the world hardest hit by HIV where access to lifelong medication is far from guaranteed.
These two studies are not the end of the road, but they mark a crucial turn. They demonstrate that the immune system can be shown where to look and how to fight—if we design our vaccines with enough precision, realism, and biological insight.
It’s not just about clever molecules. It’s about a shift in philosophy. Rather than overwhelm the immune system with brute force, we are now learning to guide it—one mRNA strand at a time—toward the exact vulnerabilities of one of the world’s most elusive viruses.
If the early signs hold, the long quest for an HIV vaccine may finally be gaining momentum. And with it, a glimmer of hope that we might one day speak of AIDS not as a global crisis, but as a vanquished chapter of medical history.
More information: Parham Ramezani-Rad et al, Vaccination with an mRNA-encoded membrane-bound HIV envelope trimer induces neutralizing antibodies in animal models, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.adw0721
K. Rachael Parks et al, Vaccination with mRNA-encoded membrane-anchored HIV envelope trimers elicited tier 2 neutralizing antibodies in a phase 1 clinical trial, Science Translational Medicine (2025). DOI: 10.1126/scitranslmed.ady6831