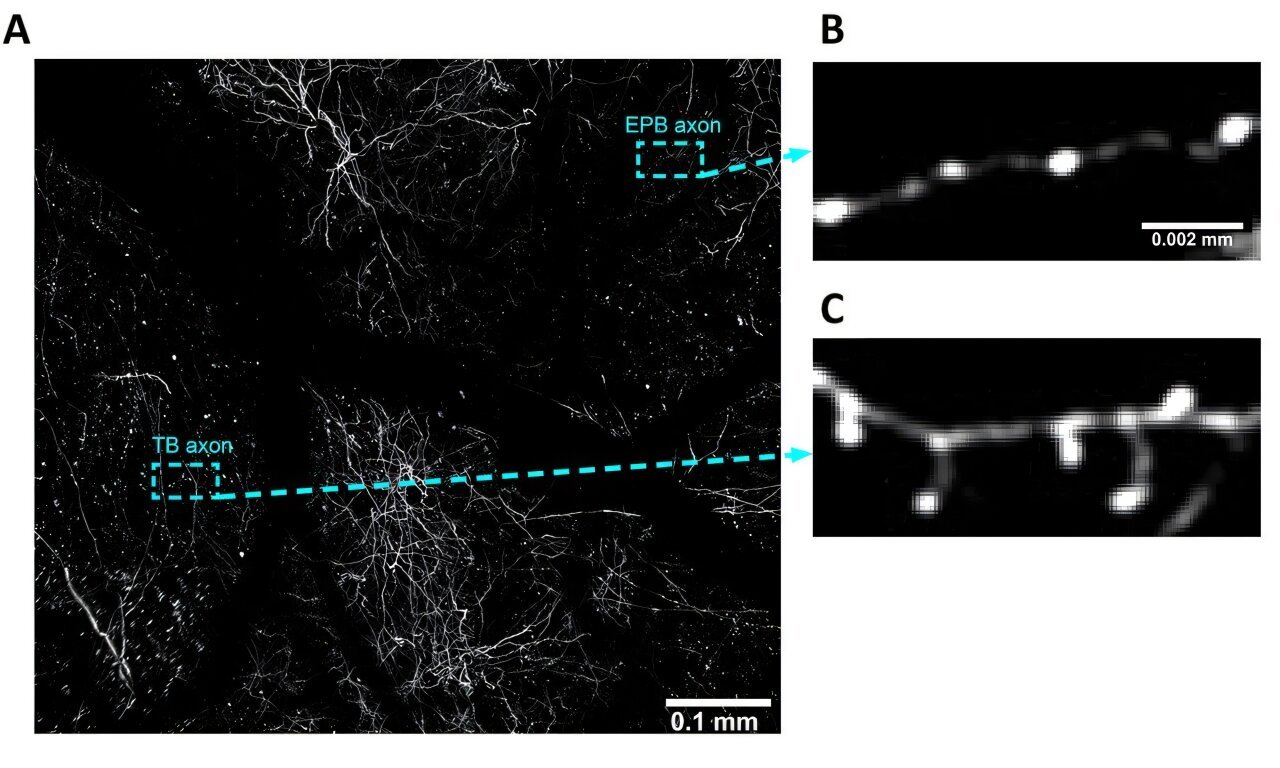
The human brain—our species’ crowning biological achievement—is an intricate cathedral of thought, memory, and imagination. Among its most awe-inspiring structures is the cerebral cortex, a folded expanse of gray matter that orchestrates everything from language to abstract reasoning. Compared to our closest evolutionary relatives, the human cortex is strikingly larger and more complex, a feature that scientists believe underpins our unique cognitive capabilities. But how, precisely, did nature sculpt this organ into such a potent seat of intellect?
A new study led by researchers at Duke University Medical Center brings us closer to answering that question. Published in Nature under the title “A human-specific enhancer fine-tunes radial glia potency and corticogenesis,” the research zeroes in on a tiny but mighty piece of non-coding DNA—a region once thought of as “junk”—called HARE5. This regulatory sequence is not a gene, but rather a genetic enhancer that acts like a dimmer switch, dialing up the activity of genes critical to brain development.
And in humans, that dimmer has been turned up.
The Human Accelerated Regions: A Genomic Treasure Hunt
The story of HARE5 begins with the discovery of Human Accelerated Regions (HARs)—segments of DNA that have remained relatively unchanged throughout mammalian evolution, only to suddenly accumulate rapid mutations in humans after our split from chimpanzees. These regions don’t code for proteins themselves, but they are often nestled near genes involved in brain development.
Of the thousands of HARs now cataloged, many cluster around genes that guide the growth and differentiation of neural cells. They are the subtle genetic brushstrokes that, over millennia, may have painted the human brain into its modern form. But which of these brushstrokes are meaningful? And how do they work?
To explore this question, researchers focused on HARE5, located on chromosome 10 near the gene FZD8, which encodes a receptor in the WNT signaling pathway—a pathway deeply embedded in the developmental choreography of the cerebral cortex.
The FZD8 Connection: Wiring the Brain’s Growth Pathways
The WNT signaling cascade is among the brain’s most influential pathways during development. It controls the balance between neural progenitor proliferation (the generation of new precursor cells) and differentiation (their transformation into specialized neurons). FZD8, a WNT receptor, plays a critical role in this process, acting as a molecular switchboard that interprets environmental cues and shapes the architecture of the developing cortex.
The Duke researchers hypothesized that HARE5—located just upstream of FZD8—might influence how this gene is expressed during brain formation. Intriguingly, human-specific changes in HARE5 could make the enhancer more potent, leading to more active FZD8 signaling, faster or longer-lasting progenitor cell division, and, ultimately, a bigger, more neuron-dense cortex.
To test this idea, the team embarked on a genetic detective mission using an arsenal of modern tools: CRISPR genome editing, stem cell reprogramming, cortical organoids, live imaging, single-cell RNA sequencing, and functional brain imaging in transgenic mice.
Mice With Human Enhancers: Rewiring the Rodent Brain
Using CRISPR, the team engineered mouse embryos to carry either the human, chimpanzee, or mouse versions of HARE5 upstream of the mouse Fzd8 gene. This approach created genetically controlled environments in which any observed differences in brain development could be directly attributed to the enhancer’s sequence.
The results were astonishing.
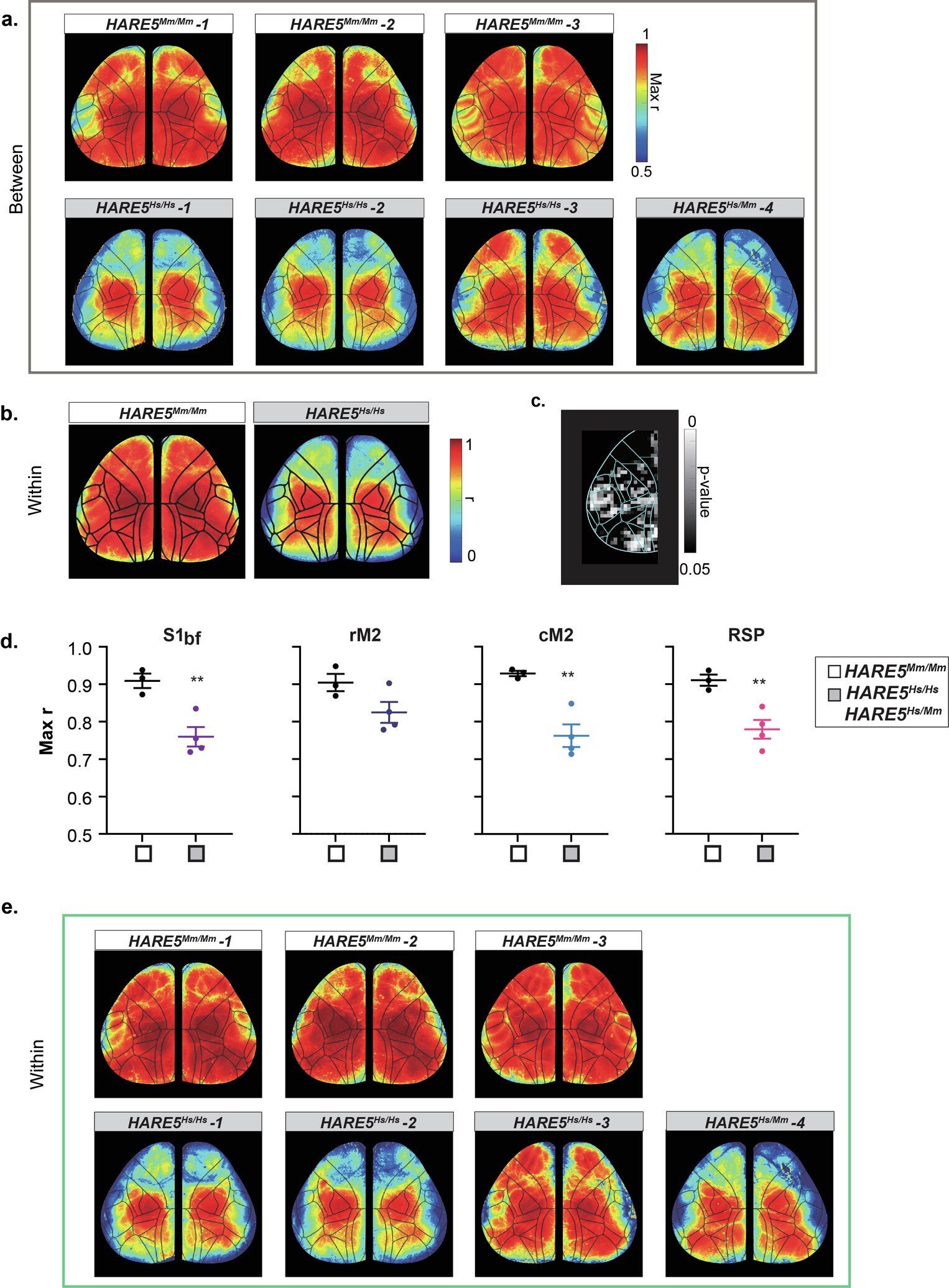
Mice carrying the human HARE5 sequence developed larger cortices than their chimpanzee- and mouse-enhancer counterparts. These mice exhibited significantly more mature neurons—especially in the upper cortical layers associated with complex sensory processing and cognition. Their brains, while still fundamentally rodent in shape and size, bore subtle but measurable shifts toward human-like complexity.
The expansion wasn’t random. It was driven by more robust proliferation and delayed differentiation of radial glial cells, the stem-like neural progenitors that act as scaffolds and neuron factories during brain development. With human HARE5 at the helm, these cells divided more frequently and retained their progenitor identity longer—delivering more neurons to the growing cortex.
Inside the Enhancer: Cracking the Genetic Code
But what was it about the human version of HARE5 that made it such a powerful enhancer?
To find out, the team dissected the enhancer down to its individual nucleotides. They discovered that four specific human-only substitutions were primarily responsible for the increased activity. Two variants—referred to as Variant I and Variant II—accounted for roughly 80% of the enhancer’s functional boost.
By swapping these variants into the chimpanzee version of HARE5, researchers could replicate much of the human enhancer’s behavior. Conversely, mutating them in the human version suppressed enhancer activity, reinforcing their critical role.
Organoids in a Dish: Recreating the Brain’s Genesis
Beyond mice, the team applied the same enhancer modifications to human embryonic stem cells and chimpanzee induced pluripotent stem cells. From these, they grew cortical organoids—tiny, spherical brain-like structures that mimic the early developmental stages of the human cortex.
In these organoids, cells with human HARE5 showed elevated expression of FZD8 and greater neural progenitor proliferation. Organoids grew larger and harbored more neurons, further validating the enhancer’s function across species and systems.
Live imaging of these growing brainlets showed accelerated radial glial cell division. Lineage tracing revealed more symmetrical divisions, a hallmark of self-renewing stem cell behavior, and a developmental trajectory that allowed more neurons to be generated later on.
A Connection to Autism: The Dark Side of Enhancement
The power of HARE5 to sculpt the brain is awe-inspiring—but also double-edged. The researchers found that mutations associated with autism spectrum disorder (ASD) located adjacent to Variant I significantly reduced enhancer activity. These mutations correlated with diminished progenitor proliferation and smaller cortical structures.
This suggests that even subtle alterations in enhancer regions like HARE5 can disrupt the delicate balance of neurodevelopment—potentially leading to cognitive disorders or developmental delays. Importantly, this links non-coding DNA—not just mutations in protein-coding genes—to conditions like autism.
CRISPR Interference: Proving Causality
To confirm that HARE5’s effect on brain growth was mediated through Fzd8, the team employed CRISPR interference (CRISPRi). This technique allowed them to selectively silence the enhancer in neural progenitor cells without altering the Fzd8 gene itself.
The result? A sharp decrease in Fzd8 expression and progenitor proliferation, proving HARE5’s regulatory power was both necessary and specific. When HARE5 was knocked out conditionally in mice, cortical size dropped significantly, and neurogenesis waned—further confirming its role in shaping the architecture of the cortex.
Measuring the Brain’s Orchestra: Calcium Imaging and Neural Networks
Beyond the cellular level, the researchers wanted to know: do these structural changes translate to functional differences in brain activity?
Using wide-field calcium imaging, which tracks neural activity across the cortex, they observed increased functional independence between brain regions in mice with human HARE5. This suggests more modular and potentially more specialized networks—features associated with human cognition.
While mouse brains cannot replicate human thought, these network-level shifts offer tantalizing clues. HARE5 may not just build a bigger brain—it may help wire it for more sophisticated computation.
Redefining the Genome: From Genes to Enhancers
This study represents a milestone in our understanding of how non-coding DNA sequences—once dismissed as “junk”—play essential roles in evolutionary innovation. Enhancers like HARE5 don’t build proteins; they orchestrate development, tuning when and where genes are expressed and at what intensity.
In evolution, it’s not just the components that matter—it’s how they’re arranged and activated. HARE5 proves that tiny tweaks in regulatory DNA can yield profound biological consequences. In this case, four human-specific mutations helped shift the trajectory of cortical development, increasing the brain’s size and neuron count in ways that echo the evolutionary path of Homo sapiens.
Looking Forward: The Future of Brain Genomics
The implications of this research are vast. By identifying how individual nucleotide changes affect brain growth and structure, scientists gain a powerful new framework for studying neurodevelopmental disorders. Conditions like autism, schizophrenia, and intellectual disability may be driven not only by mutations in genes, but also by hidden glitches in their regulatory partners.
Moreover, this study opens new avenues in the field of evolutionary developmental biology (evo-devo). Future research may explore whether other HARs exert similar effects, potentially uncovering a genomic atlas of human brain evolution.
Could we one day trace the rise of language, creativity, or consciousness to a handful of enhancers? Could personalized medicine use enhancer profiles to predict or treat cognitive disorders? These are no longer science fiction questions—they are the new frontier.
Conclusion: A Tiny Switch with Titanic Consequences
In the vast library of the human genome, HARE5 is just one of many enhancers—an editorial note in the margins of our biological script. But its story is emblematic of a deeper truth: the architecture of the human mind is not built by genes alone, but by the intricate choreography of their regulation.
By decoding how a few molecular edits in HARE5 helped shape the human cortex, scientists are beginning to unravel the blueprint behind our greatest evolutionary leap—the birth of a brain capable of understanding its own origins.
And as we probe these ancient instructions, we may find not just the roots of intelligence, but the keys to healing its disorders and expanding its horizons.
Reference: Jing Liu et al, A human-specific enhancer fine-tunes radial glia potency and corticogenesis, Nature (2025). DOI: 10.1038/s41586-025-09002-1