The mystery began with a contradiction that has haunted psychiatry for decades. Schizophrenia, a disorder marked by hallucinations, delusions, and profound shifts in thought and perception, often begins to show itself in early adulthood. Yet many of the genetic risk factors linked to it appear to act far earlier, during the earliest stages of brain development. This temporal gap—between the origins of risk and the moment symptoms surface—has long puzzled researchers.
“Our work began with a longstanding question in psychiatry and neuroscience: why do genetic risk factors for schizophrenia, many of which act during early brain development—manifest clinically much later in life?” said Kiran Girdhar and Panos Roussos.
For scientists at the Center for Disease Neurogenomics and the Friedman Brain Institute at the Icahn School of Medicine at Mount Sinai, the answer might lie buried inside the quiet architecture of chromatin, the molecular packaging that wraps DNA. Chromatin can be loose or tight, open or closed, and these configurations determine whether genes can be switched on or off. They are marks of epigenetic memory, hints of past developmental states that might persist far longer than anyone realized.
A Thousand Brains and a Search for Hidden Signatures
To trace these epigenetic echoes, the team embarked on an ambitious project. They collected postmortem brain samples from more than a thousand individuals, with and without schizophrenia. Their focus was the prefrontal cortex, a region central to reasoning, planning, and other higher cognitive functions that often falter in the disorder.
The researchers meticulously separated the brain nuclei into two groups: those belonging to neurons and those belonging to non-neuronal cells. This separation was essential. A single brain contains a mix of many cell types, and if their molecular signals blur together, the most crucial differences might be lost.
Their next step required a technique known as ATAC-seq, which inserts small molecular tags into regions of open chromatin. Where the chromatin is open, the tags slip in easily, marking areas where gene regulation may be active. Layer by layer, sample by sample, these tags created one of the most detailed chromatin accessibility maps ever made in schizophrenia research—spanning more than 1,300 brain samples.
This map was not simply a catalog. It was a narrative, a way of reading how gene regulation differs between those with schizophrenia and those without.
Where the Genome Whispers Its Clues
As the researchers compared the chromatin landscapes, a striking pattern emerged. Neuronal regions that showed increased chromatin accessibility in individuals with schizophrenia were also enriched with known genetic risk variants. This was not true for non-neuronal regions. Something specific was happening inside neurons, something tightly tied to the disorder.
By integrating these chromatin maps with genetic and single-cell data, the team uncovered a deeper layer of the story. Regions of open chromatin in adult neurons with schizophrenia resembled patterns usually seen during fetal brain development. These were not random similarities; they were signatures of early-life processes lingering into adulthood.
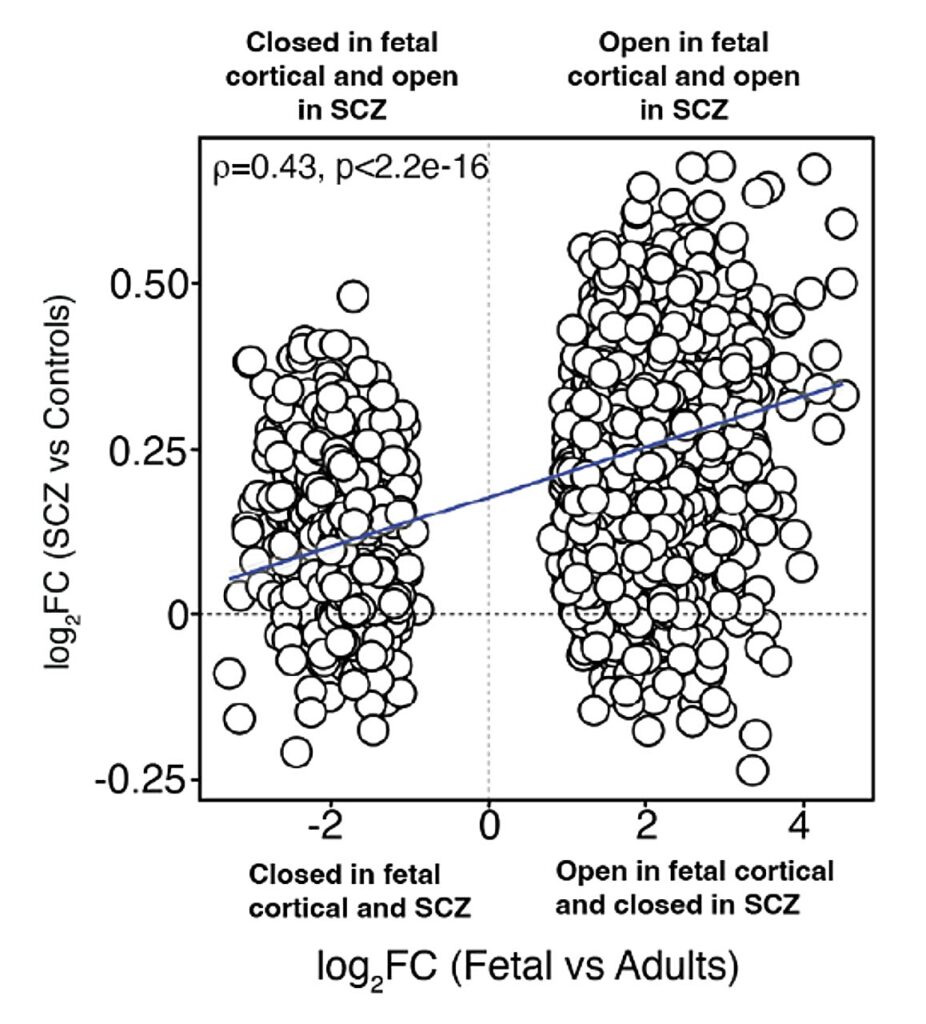
“The chromatin patterns we observed in adult neurons with schizophrenia mirror those found in the developing fetal brain,” explained Girdhar and Roussos. “This provides a molecular bridge between early developmental processes and the adult disease state.”
This discovery suggested that the epigenetic marks laid down in early development—marks shaped by genetic risk—do not vanish as the brain matures. They persist quietly, waiting for the right moment, or perhaps the wrong one, to influence the adult brain.
A Genomic Hub With a Hidden Influence
Inside this developmental echo, the researchers found something even more unexpected: a neuronal trans-regulatory domain. Unlike typical regulatory systems, where control elements sit near the genes they influence, this domain functioned like a distant communication network. Genomic regions scattered across chromosomes appeared to act together, coordinating gene activity from afar.
This hub was especially enriched in immature glutamatergic neurons, a type of neuron involved in excitatory signaling. It concentrated the same neurodevelopmental chromatin signatures that marked schizophrenia risk, acting like a central switchboard where many distant signals converged.
To test the importance of this hub, the team examined whether genetic variants located within it could distinguish individuals with schizophrenia from those without. Although these variants represented only a small fraction—just 2 to 3 percent—of all known risk variants, they reliably predicted disease status in a massive independent cohort of more than 195,000 individuals from the Million Veteran Program.
It was a rare moment in psychiatric genomics: a small set of molecular clues pointing with surprising accuracy to a complex disorder.
Toward a New Map of the Illness
“This study provides one of the most detailed maps to date of neuronal and non-neuronal chromatin accessibility map in the context of schizophrenia,” said Girdhar and Roussos. “It shows that the molecular architecture of the disease is deeply rooted in early brain development, even though symptoms appear much later.”
But for the team, this is only the beginning. Their next goal is to refine this map further, moving beyond the broad category of “neurons” to identify the specific neuronal subtypes and cortical layers where the most crucial chromatin changes occur. They envision combining single-cell ATAC-seq with spatial mapping, weaving together chromatin data with single-cell RNA and protein information.
The result could be a multilayered portrait of schizophrenia risk: which genomic regions open, in which types of cells, in which layers of the cortex, and with which downstream effects on gene and protein function.
Their ultimate aim is bold yet clear: to build a comprehensive, cell-type–specific regulatory map precise enough to point directly to therapeutic targets.
Why This Research Matters
Schizophrenia has long remained a scientific enigma because its origins stretch across time, from fetal development to adulthood, and across biological layers, from genes to epigenetic regulation to neural circuits. This study offers one of the clearest molecular explanations yet for how early genetic risk factors might leave traces that persist into adult life.
By uncovering chromatin patterns that bridge fetal development and adult illness, the research reframes schizophrenia not as a sudden onset disorder but as a lifelong biological narrative, written quietly in the genome and revealed only years later.
This work does more than illuminate the roots of a complex condition. It offers a roadmap for future therapies—therapies grounded in understanding not just where the brain goes wrong, but when, and how its earliest instructions echo across a lifetime.
More information: Kiran Girdhar et al, The neuronal chromatin landscape in brains from individuals with schizophrenia is linked to early fetal development, Nature Neuroscience (2025). DOI: 10.1038/s41593-025-02081-3.